1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 0927-2014 English PDF (YY/T0927-2014)
YY/T 0927-2014 English PDF (YY/T0927-2014)
Regular price
$150.00
Regular price
Sale price
$150.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 0927-2014: Guidance of determination for di(2-ethylhexyl)phthalate (DEHP) released from PVC medical devices
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click YY/T 0927-2014 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0927-2014
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0927-2014
GB
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
聚氯乙烯医疗器械中邻苯二甲酸二
ISSUED ON. JUNE 17, 2014
IMPLEMENTED ON. JULY 1, 2015
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 6
2 Normative references ... 6
3 General principles ... 6
4 Principles for extract solution preparation ... 6
5 Determination of DEHP release by gas chromatography / mass
spectrometer method (GC/MS) ... 8
6 Determination of DEHP release by UV method ... 12
Introduction
Di (2-ethylhexyl) phthalate (DEHP) is one of the commonly used plasticizers for
PVC disposable medical devices. It can improve the flexibility and cold
resistance of the plastic, lower the softening temperature, and improve the
processing performance.
Potential side effects produced by DEHP and other phthalate esters have been
widely discussed in the scientific community, then controversy has been
sparked. Although DEHP’s toxicity, its teratogenic and carcinogenic effects
have been well proved in laboratory animals, however, whether it can produce
the same effect to human body is still controversial.
At present, allowable limits on human body of DEHP have been studied with
results. The international standards concerning allowable limits for related
medical devices are under development. During the use of devices, the amount
of DEHP received by patients is related to clinical contact manner. Therefore, it
is impossible for this standard to offer an analysis method which applies to all
contact manners. Because of this, it suggests in this standard that it shall use
stimulation of clinical method to prepare extract solution.
There are a variety of analytical methods to determine DEHP release. Typical
methods include gas chromatography (GC), high performance liquid
chromatography (HPLC), gas chromatography / mass spectrometer method
(GC/MS), liquid chromatography / mass spectrometer method (LC-MS), and
UV-visible spectrophotometry. This standard takes gas chromatography / mass
spectrometer method (GC/MS) and UV-visible spectrophotometry as the basic
methods. And test procedures are offered.
Since there is a wide range of PVC medical devices, different devices have very
different clinical applications; under some situations, the published literature
methods including the methods offered in this standard do not apply to all
devices, any analytical method shall be used as long as it is proved as
analytically reliable.
“Analytically reliable” refers to the method used has sufficient precision,
accuracy, linearity, sensitivity and selectivity, when it analyzes DEHP release
for extract solution obtained by PVC devices under certain extraction medium
and conditions.
DEHP is liposoluble in PVC devices. When infusing drug lipids, blood or blood
components, it shall conduct DEHP release test, so as to determine if PVC
devices are suitable for infusion of particular liquid. And evaluate it according to
risk management requirements.
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
1 Scope
This standard provides the chemical analysis methods for release of plasticizer
DEHP after medical device which takes PVC as raw material is contacting with
liquid of clinical use, under simulation of actual conditions of use.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 6682 Water for analytical laboratory use - Specification and test
methods
3 General principles
3.1 Unless otherwise specified, room temperature in this standard shall be
10°C~30°C.
3.2 Test water in this standard shall meet requirements in GB/T 6682.
3.3 Unless otherwise specified, container used in this standard shall be
borosilicate glass container.
3.4 It shall use the pipette which meets accuracy requirements specified in
corresponding national standards to measure.
4 Principles for extract solution preparation
4.1 General principle
The preparation of DEHP extract solution in PVC medical devices shall consider
the clinical usage of those devices, then choose appropriate method for extract
solution preparation (e.g., extract solvent, time, temperature and mode of
hexane to dilute it to 10mL, and standard stock solution of which concentration
is about 2000 μg/mL shall be obtained (store it in the fridge at 2°C~10°C; 1
month validity).
5.4.2 Take standard stock solution mentioned above. Take hexane as solvent.
Adopt serial dilution to prepare 5 working standard solutions of which
concentration is 0.5 μg/mL~200 μg/mL (store it in the fridge at 2°C~10°C; 1
month validity).
5.5 Pre-treatment of sample extract solution
5.5.1 Pre-treatment of blood-contacting-device extract solution
5.5.1.1 Extract solution which takes ethanol / water mixture as extract
solvent
Take certain volume of extract solution prepared in 4.2.1 to dry in vacuum at
50°C. After dying, cool it to room temperature. Then add hexane of equivalent
volume. Scroll and dissolve it for 1 min to obtain inspection solution.
5.5.1.2 Extract solution which takes blood or blood components as extract
solvent
Take 0.5 mL of extract solution prepared in 4.2.2, and put it in centrifuge tube.
Add 2 mL of hexane solvent. Scroll, mix and extract for 1 min; centrifuge for 10
min at 3000 times/min. Take supernatant liquid as the inspection solution.
5.5.2 Extract solution which takes sodium chloride injection and/or
glucose injection diluted lipid drugs as extract solvent
Take certain volume of extract solution prepared in 4.3 to dry in vacuum at 50°C.
After dying, cool it to room temperature. Then add hexane of equivalent volume.
Use vortex mixer to scroll and dissolve it for about 1 min. Take supernatant
liquid as inspection solution.
Note. Due to a wide variety of clinical drug infusion, when conduct determination of DEHP
release by referring to the conditions in 5.6, it shall verify the methodology which is close
to it, for example, the main ingredients of drug shall not interfere the determination of DEHP,
the recovery rate near measured DEHP released concentration, etc.
5.5.3 Extract solution of other extract solvents
It shall determine the pre-treatment method of extract solution according to the
selected extract solvent combined with necessary methodological research, so
as to demonstrate the reliability of analysis method.
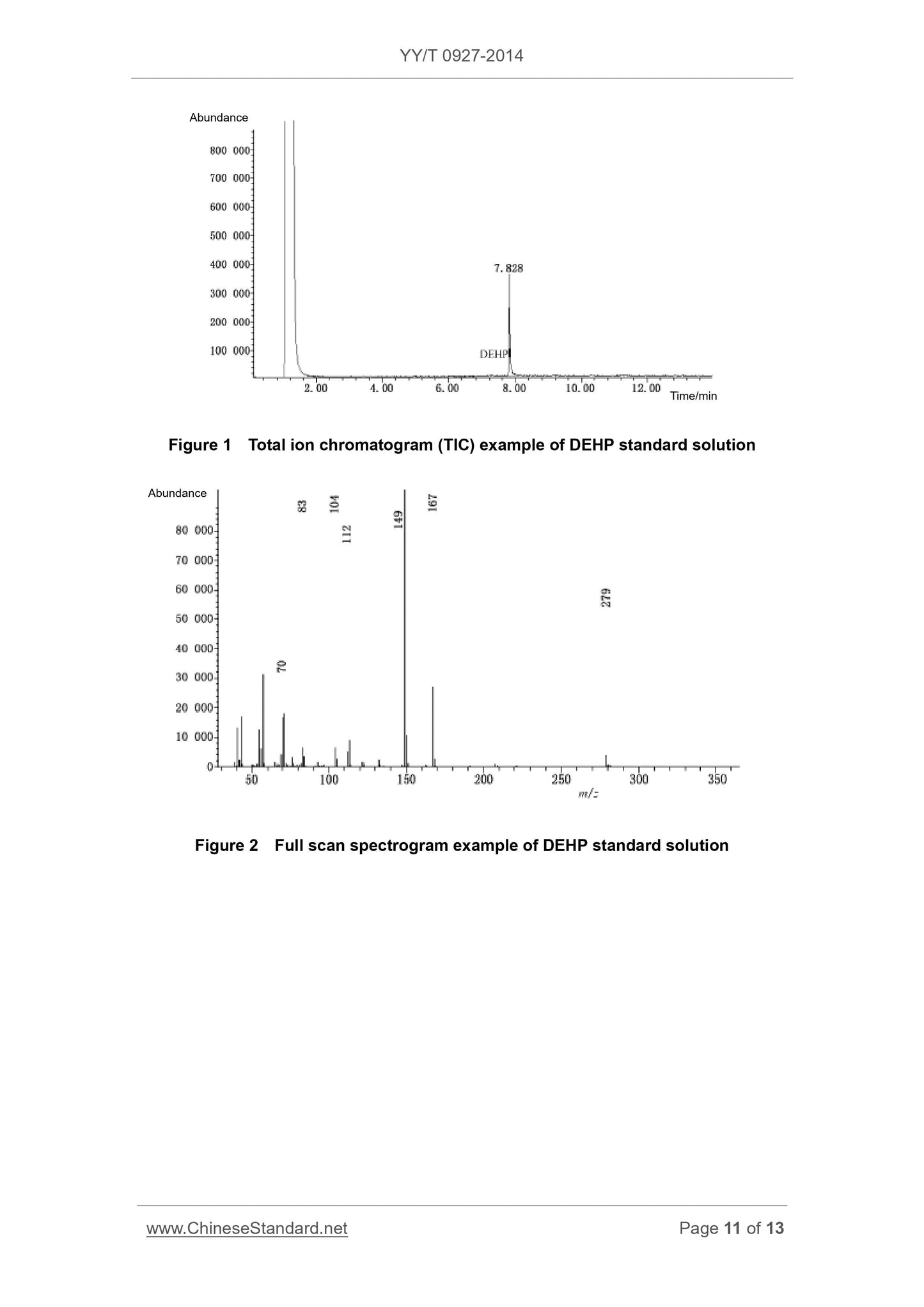
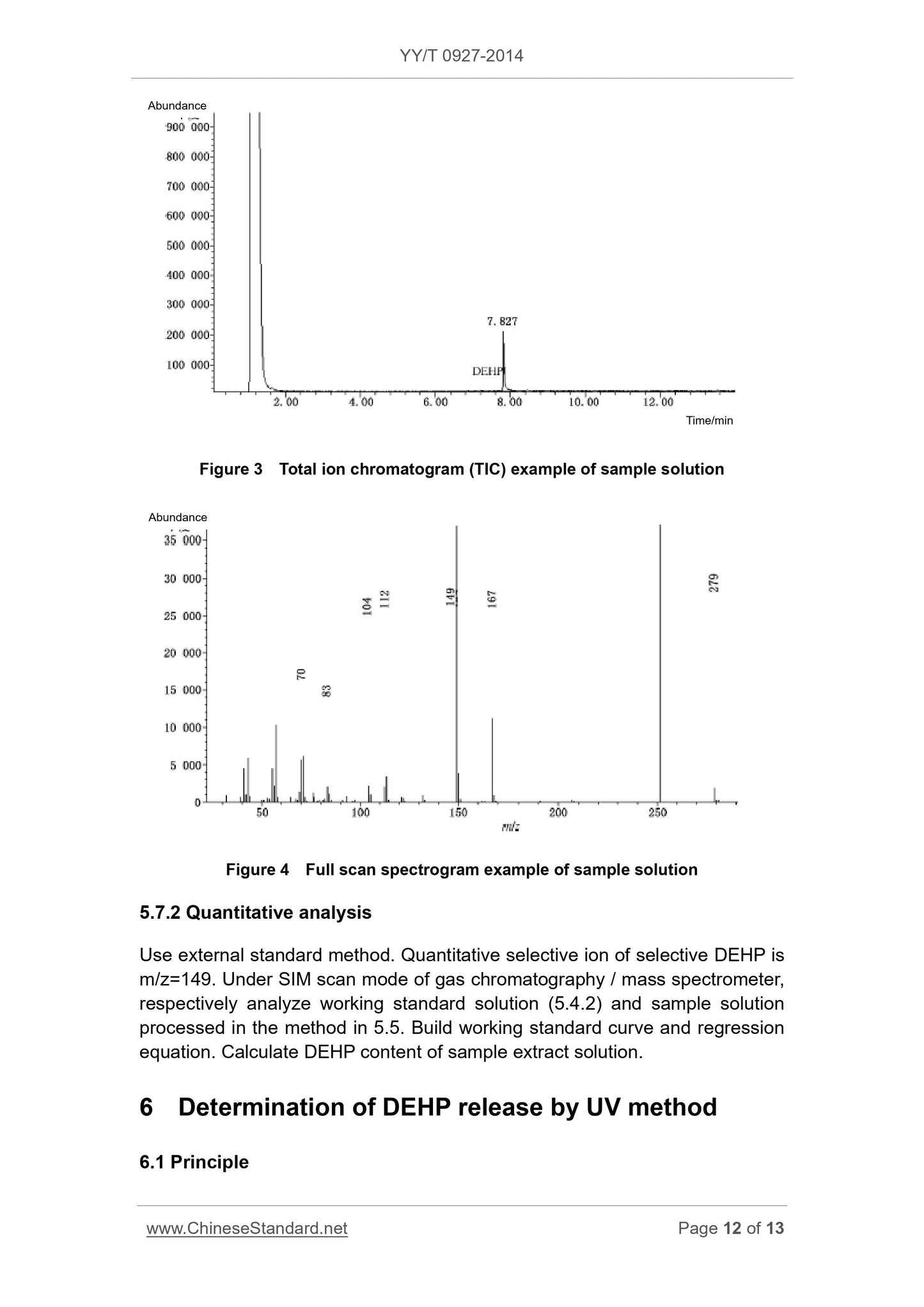
5.6 GC / MS conditions (recommendatory)
Prepare DEHP working standard solution. Use UV spectrophotometry to obtain
working curve and regression equation of standard solution. Determine sample
solution in same method. Use regression equation to quantify DEHP in sample
solution.
Note. This method is only applicable to the sample solution which takes ethanol
water solution as extract solvent. When product components contain other non-
PVC material, it may interfere the determination.
6.2 Preparation of working standard solution
6.2.1 Use liquid specific gravity balance (or device of equivalent accuracy) to
prepare ethanol solution of which density is 0.8050 g/mL~0.8123 g/mL (at 20°C).
6.2.2 Take about 1 g of standard DEHP (make it accurate to 0.1 mg). Use
ethanol solution (density is 0.8050 g/mL~0.8123 g/mL) to dilute it to 100 mL.
And standard stock solution of which concentration is 10000 μg/mL shall be
obtained.
6.2.3 Use ethanol solution (density is 0.8050 g/mL~0.8123 g/mL) as solvent to
prepare at least 5 working standard solutions of which concentration is 10
μg/mL~100 μg/mL.
6.3 Determination and result calculation
6.3.1 At 272 nm, use extract solvent (refer to 4.2.1) as reference solution to
measure absorbance values of standard solution, and obtain working curve and
regression equation.
6.3.2 Take extract solution prepared according to 4.2. Measure absorbance
values of sample solution at 272 nm. Calculate DEHP content by regression
equation.
YY/T 0927-2014
GB
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
聚氯乙烯医疗器械中邻苯二甲酸二
ISSUED ON. JUNE 17, 2014
IMPLEMENTED ON. JULY 1, 2015
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 6
2 Normative references ... 6
3 General principles ... 6
4 Principles for extract solution preparation ... 6
5 Determination of DEHP release by gas chromatography / mass
spectrometer method (GC/MS) ... 8
6 Determination of DEHP release by UV method ... 12
Introduction
Di (2-ethylhexyl) phthalate (DEHP) is one of the commonly used plasticizers for
PVC disposable medical devices. It can improve the flexibility and cold
resistance of the plastic, lower the softening temperature, and improve the
processing performance.
Potential side effects produced by DEHP and other phthalate esters have been
widely discussed in the scientific community, then controversy has been
sparked. Although DEHP’s toxicity, its teratogenic and carcinogenic effects
have been well proved in laboratory animals, however, whether it can produce
the same effect to human body is still controversial.
At present, allowable limits on human body of DEHP have been studied with
results. The international standards concerning allowable limits for related
medical devices are under development. During the use of devices, the amount
of DEHP received by patients is related to clinical contact manner. Therefore, it
is impossible for this standard to offer an analysis method which applies to all
contact manners. Because of this, it suggests in this standard that it shall use
stimulation of clinical method to prepare extract solution.
There are a variety of analytical methods to determine DEHP release. Typical
methods include gas chromatography (GC), high performance liquid
chromatography (HPLC), gas chromatography / mass spectrometer method
(GC/MS), liquid chromatography / mass spectrometer method (LC-MS), and
UV-visible spectrophotometry. This standard takes gas chromatography / mass
spectrometer method (GC/MS) and UV-visible spectrophotometry as the basic
methods. And test procedures are offered.
Since there is a wide range of PVC medical devices, different devices have very
different clinical applications; under some situations, the published literature
methods including the methods offered in this standard do not apply to all
devices, any analytical method shall be used as long as it is proved as
analytically reliable.
“Analytically reliable” refers to the method used has sufficient precision,
accuracy, linearity, sensitivity and selectivity, when it analyzes DEHP release
for extract solution obtained by PVC devices under certain extraction medium
and conditions.
DEHP is liposoluble in PVC devices. When infusing drug lipids, blood or blood
components, it shall conduct DEHP release test, so as to determine if PVC
devices are suitable for infusion of particular liquid. And evaluate it according to
risk management requirements.
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
1 Scope
This standard provides the chemical analysis methods for release of plasticizer
DEHP after medical device which takes PVC as raw material is contacting with
liquid of clinical use, under simulation of actual conditions of use.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 6682 Water for analytical laboratory use - Specification and test
methods
3 General principles
3.1 Unless otherwise specified, room temperature in this standard shall be
10°C~30°C.
3.2 Test water in this standard shall meet requirements in GB/T 6682.
3.3 Unless otherwise specified, container used in this standard shall be
borosilicate glass container.
3.4 It shall use the pipette which meets accuracy requirements specified in
corresponding national standards to measure.
4 Principles for extract solution preparation
4.1 General principle
The preparation of DEHP extract solution in PVC medical devices shall consider
the clinical usage of those devices, then choose appropriate method for extract
solution preparation (e.g., extract solvent, time, temperature and mode of
hexane to dilute it to 10mL, and standard stock solution of which concentration
is about 2000 μg/mL shall be obtained (store it in the fridge at 2°C~10°C; 1
month validity).
5.4.2 Take standard stock solution mentioned above. Take hexane as solvent.
Adopt serial dilution to prepare 5 working standard solutions of which
concentration is 0.5 μg/mL~200 μg/mL (store it in the fridge at 2°C~10°C; 1
month validity).
5.5 Pre-treatment of sample extract solution
5.5.1 Pre-treatment of blood-contacting-device extract solution
5.5.1.1 Extract solution which takes ethanol / water mixture as extract
solvent
Take certain volume of extract solution prepared in 4.2.1 to dry in vacuum at
50°C. After dying, cool it to room temperature. Then add hexane of equivalent
volume. Scroll and dissolve it for 1 min to obtain inspection solution.
5.5.1.2 Extract solution which takes blood or blood components as extract
solvent
Take 0.5 mL of extract solution prepared in 4.2.2, and put it in centrifuge tube.
Add 2 mL of hexane solvent. Scroll, mix and extract for 1 min; centrifuge for 10
min at 3000 times/min. Take supernatant liqui...
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click YY/T 0927-2014 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0927-2014
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0927-2014
GB
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
聚氯乙烯医疗器械中邻苯二甲酸二
ISSUED ON. JUNE 17, 2014
IMPLEMENTED ON. JULY 1, 2015
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 6
2 Normative references ... 6
3 General principles ... 6
4 Principles for extract solution preparation ... 6
5 Determination of DEHP release by gas chromatography / mass
spectrometer method (GC/MS) ... 8
6 Determination of DEHP release by UV method ... 12
Introduction
Di (2-ethylhexyl) phthalate (DEHP) is one of the commonly used plasticizers for
PVC disposable medical devices. It can improve the flexibility and cold
resistance of the plastic, lower the softening temperature, and improve the
processing performance.
Potential side effects produced by DEHP and other phthalate esters have been
widely discussed in the scientific community, then controversy has been
sparked. Although DEHP’s toxicity, its teratogenic and carcinogenic effects
have been well proved in laboratory animals, however, whether it can produce
the same effect to human body is still controversial.
At present, allowable limits on human body of DEHP have been studied with
results. The international standards concerning allowable limits for related
medical devices are under development. During the use of devices, the amount
of DEHP received by patients is related to clinical contact manner. Therefore, it
is impossible for this standard to offer an analysis method which applies to all
contact manners. Because of this, it suggests in this standard that it shall use
stimulation of clinical method to prepare extract solution.
There are a variety of analytical methods to determine DEHP release. Typical
methods include gas chromatography (GC), high performance liquid
chromatography (HPLC), gas chromatography / mass spectrometer method
(GC/MS), liquid chromatography / mass spectrometer method (LC-MS), and
UV-visible spectrophotometry. This standard takes gas chromatography / mass
spectrometer method (GC/MS) and UV-visible spectrophotometry as the basic
methods. And test procedures are offered.
Since there is a wide range of PVC medical devices, different devices have very
different clinical applications; under some situations, the published literature
methods including the methods offered in this standard do not apply to all
devices, any analytical method shall be used as long as it is proved as
analytically reliable.
“Analytically reliable” refers to the method used has sufficient precision,
accuracy, linearity, sensitivity and selectivity, when it analyzes DEHP release
for extract solution obtained by PVC devices under certain extraction medium
and conditions.
DEHP is liposoluble in PVC devices. When infusing drug lipids, blood or blood
components, it shall conduct DEHP release test, so as to determine if PVC
devices are suitable for infusion of particular liquid. And evaluate it according to
risk management requirements.
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
1 Scope
This standard provides the chemical analysis methods for release of plasticizer
DEHP after medical device which takes PVC as raw material is contacting with
liquid of clinical use, under simulation of actual conditions of use.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 6682 Water for analytical laboratory use - Specification and test
methods
3 General principles
3.1 Unless otherwise specified, room temperature in this standard shall be
10°C~30°C.
3.2 Test water in this standard shall meet requirements in GB/T 6682.
3.3 Unless otherwise specified, container used in this standard shall be
borosilicate glass container.
3.4 It shall use the pipette which meets accuracy requirements specified in
corresponding national standards to measure.
4 Principles for extract solution preparation
4.1 General principle
The preparation of DEHP extract solution in PVC medical devices shall consider
the clinical usage of those devices, then choose appropriate method for extract
solution preparation (e.g., extract solvent, time, temperature and mode of
hexane to dilute it to 10mL, and standard stock solution of which concentration
is about 2000 μg/mL shall be obtained (store it in the fridge at 2°C~10°C; 1
month validity).
5.4.2 Take standard stock solution mentioned above. Take hexane as solvent.
Adopt serial dilution to prepare 5 working standard solutions of which
concentration is 0.5 μg/mL~200 μg/mL (store it in the fridge at 2°C~10°C; 1
month validity).
5.5 Pre-treatment of sample extract solution
5.5.1 Pre-treatment of blood-contacting-device extract solution
5.5.1.1 Extract solution which takes ethanol / water mixture as extract
solvent
Take certain volume of extract solution prepared in 4.2.1 to dry in vacuum at
50°C. After dying, cool it to room temperature. Then add hexane of equivalent
volume. Scroll and dissolve it for 1 min to obtain inspection solution.
5.5.1.2 Extract solution which takes blood or blood components as extract
solvent
Take 0.5 mL of extract solution prepared in 4.2.2, and put it in centrifuge tube.
Add 2 mL of hexane solvent. Scroll, mix and extract for 1 min; centrifuge for 10
min at 3000 times/min. Take supernatant liquid as the inspection solution.
5.5.2 Extract solution which takes sodium chloride injection and/or
glucose injection diluted lipid drugs as extract solvent
Take certain volume of extract solution prepared in 4.3 to dry in vacuum at 50°C.
After dying, cool it to room temperature. Then add hexane of equivalent volume.
Use vortex mixer to scroll and dissolve it for about 1 min. Take supernatant
liquid as inspection solution.
Note. Due to a wide variety of clinical drug infusion, when conduct determination of DEHP
release by referring to the conditions in 5.6, it shall verify the methodology which is close
to it, for example, the main ingredients of drug shall not interfere the determination of DEHP,
the recovery rate near measured DEHP released concentration, etc.
5.5.3 Extract solution of other extract solvents
It shall determine the pre-treatment method of extract solution according to the
selected extract solvent combined with necessary methodological research, so
as to demonstrate the reliability of analysis method.
5.6 GC / MS conditions (recommendatory)
Prepare DEHP working standard solution. Use UV spectrophotometry to obtain
working curve and regression equation of standard solution. Determine sample
solution in same method. Use regression equation to quantify DEHP in sample
solution.
Note. This method is only applicable to the sample solution which takes ethanol
water solution as extract solvent. When product components contain other non-
PVC material, it may interfere the determination.
6.2 Preparation of working standard solution
6.2.1 Use liquid specific gravity balance (or device of equivalent accuracy) to
prepare ethanol solution of which density is 0.8050 g/mL~0.8123 g/mL (at 20°C).
6.2.2 Take about 1 g of standard DEHP (make it accurate to 0.1 mg). Use
ethanol solution (density is 0.8050 g/mL~0.8123 g/mL) to dilute it to 100 mL.
And standard stock solution of which concentration is 10000 μg/mL shall be
obtained.
6.2.3 Use ethanol solution (density is 0.8050 g/mL~0.8123 g/mL) as solvent to
prepare at least 5 working standard solutions of which concentration is 10
μg/mL~100 μg/mL.
6.3 Determination and result calculation
6.3.1 At 272 nm, use extract solvent (refer to 4.2.1) as reference solution to
measure absorbance values of standard solution, and obtain working curve and
regression equation.
6.3.2 Take extract solution prepared according to 4.2. Measure absorbance
values of sample solution at 272 nm. Calculate DEHP content by regression
equation.
YY/T 0927-2014
GB
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
聚氯乙烯医疗器械中邻苯二甲酸二
ISSUED ON. JUNE 17, 2014
IMPLEMENTED ON. JULY 1, 2015
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 6
2 Normative references ... 6
3 General principles ... 6
4 Principles for extract solution preparation ... 6
5 Determination of DEHP release by gas chromatography / mass
spectrometer method (GC/MS) ... 8
6 Determination of DEHP release by UV method ... 12
Introduction
Di (2-ethylhexyl) phthalate (DEHP) is one of the commonly used plasticizers for
PVC disposable medical devices. It can improve the flexibility and cold
resistance of the plastic, lower the softening temperature, and improve the
processing performance.
Potential side effects produced by DEHP and other phthalate esters have been
widely discussed in the scientific community, then controversy has been
sparked. Although DEHP’s toxicity, its teratogenic and carcinogenic effects
have been well proved in laboratory animals, however, whether it can produce
the same effect to human body is still controversial.
At present, allowable limits on human body of DEHP have been studied with
results. The international standards concerning allowable limits for related
medical devices are under development. During the use of devices, the amount
of DEHP received by patients is related to clinical contact manner. Therefore, it
is impossible for this standard to offer an analysis method which applies to all
contact manners. Because of this, it suggests in this standard that it shall use
stimulation of clinical method to prepare extract solution.
There are a variety of analytical methods to determine DEHP release. Typical
methods include gas chromatography (GC), high performance liquid
chromatography (HPLC), gas chromatography / mass spectrometer method
(GC/MS), liquid chromatography / mass spectrometer method (LC-MS), and
UV-visible spectrophotometry. This standard takes gas chromatography / mass
spectrometer method (GC/MS) and UV-visible spectrophotometry as the basic
methods. And test procedures are offered.
Since there is a wide range of PVC medical devices, different devices have very
different clinical applications; under some situations, the published literature
methods including the methods offered in this standard do not apply to all
devices, any analytical method shall be used as long as it is proved as
analytically reliable.
“Analytically reliable” refers to the method used has sufficient precision,
accuracy, linearity, sensitivity and selectivity, when it analyzes DEHP release
for extract solution obtained by PVC devices under certain extraction medium
and conditions.
DEHP is liposoluble in PVC devices. When infusing drug lipids, blood or blood
components, it shall conduct DEHP release test, so as to determine if PVC
devices are suitable for infusion of particular liquid. And evaluate it according to
risk management requirements.
Guidance of determination for di (2-ethylhexyl)
phthalate (DEHP) released from PVC medical devices
1 Scope
This standard provides the chemical analysis methods for release of plasticizer
DEHP after medical device which takes PVC as raw material is contacting with
liquid of clinical use, under simulation of actual conditions of use.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 6682 Water for analytical laboratory use - Specification and test
methods
3 General principles
3.1 Unless otherwise specified, room temperature in this standard shall be
10°C~30°C.
3.2 Test water in this standard shall meet requirements in GB/T 6682.
3.3 Unless otherwise specified, container used in this standard shall be
borosilicate glass container.
3.4 It shall use the pipette which meets accuracy requirements specified in
corresponding national standards to measure.
4 Principles for extract solution preparation
4.1 General principle
The preparation of DEHP extract solution in PVC medical devices shall consider
the clinical usage of those devices, then choose appropriate method for extract
solution preparation (e.g., extract solvent, time, temperature and mode of
hexane to dilute it to 10mL, and standard stock solution of which concentration
is about 2000 μg/mL shall be obtained (store it in the fridge at 2°C~10°C; 1
month validity).
5.4.2 Take standard stock solution mentioned above. Take hexane as solvent.
Adopt serial dilution to prepare 5 working standard solutions of which
concentration is 0.5 μg/mL~200 μg/mL (store it in the fridge at 2°C~10°C; 1
month validity).
5.5 Pre-treatment of sample extract solution
5.5.1 Pre-treatment of blood-contacting-device extract solution
5.5.1.1 Extract solution which takes ethanol / water mixture as extract
solvent
Take certain volume of extract solution prepared in 4.2.1 to dry in vacuum at
50°C. After dying, cool it to room temperature. Then add hexane of equivalent
volume. Scroll and dissolve it for 1 min to obtain inspection solution.
5.5.1.2 Extract solution which takes blood or blood components as extract
solvent
Take 0.5 mL of extract solution prepared in 4.2.2, and put it in centrifuge tube.
Add 2 mL of hexane solvent. Scroll, mix and extract for 1 min; centrifuge for 10
min at 3000 times/min. Take supernatant liqui...
Share