1
/
of
9
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0933-2022 English PDF (YYT0933-2022)
YY/T 0933-2022 English PDF (YYT0933-2022)
Regular price
$230.00 USD
Regular price
Sale price
$230.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0933-2022 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0933-2022
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0933-2022: Digital medical X-ray image detector used in general radiography
YY/T 0933-2022
Digital medical X-ray image detector used in general radiography
ICS 11.040.50
CCSC43
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0933-2014
Medical General Photography Digital X-ray Image Detector
Published on 2022-05-18
2023-06-01 Implementation
Released by the State Drug Administration
directory
Preface I
1 Scope 1
2 Normative references 1
3 Terms and Definitions 1
4 Classification and composition 2
5 Requirements 2
6 Test method 4
Appendix A (Normative) Test Layout 8
Appendix B (Informative) Test Device 10
foreword
This document is in accordance with the provisions of GB/T 1.1-2020 "Guidelines for Standardization Work Part 1.Structure and Drafting Rules of Standardization Documents"
drafted.
This document replaces YY/T 0933-2014 "Digital X-ray Image Detectors for Medical General Photography", this document and YY/T 0933-
Compared with.2014, the main technical changes are as follows.
a) Deleted the term and definition of "dose linear range" (see 3.1 of the.2014 edition);
b) Added five terms and definitions of "calibration", "central axis", "bright field image", "dark field image" and "noise equivalent dose" (see 3.1,
3.2, 3.3, 3.4 and 3.5);
c) Changed the definition of "linear dynamic range" (see 3.6, 3.2 of the.2014 edition);
d) Changed the description of "Classification", divided according to structural characteristics and data transmission (see 4.1, 4.1 of the.2014 edition);
e) Deleted "5.5 Dose Linear Range" (see 5.5 of the.2014 edition);
f) Changed the serial number of "image quality" (see 5.5, 5.7 of the.2014 edition);
g) Added "Noise Equivalent Dose" (see 5.5.1) and "Noise Equivalent Dose" test methods (see 6.5.1);
h) Changed the "linear dynamic range" (see 5.5.2, 5.6 of the.2014 edition), and added a criterion to express the degree of linearity of the gray value and dose
Fixed coefficient R2 (that is, the square value of the correlation coefficient r) (see 5.5.2), changed the "linear dynamic range" test method (see 6.5.2,
6.5 and 6.6 of the.2014 edition);
i) Changed "Line pair resolution" to "Spatial resolution" (see 5.5.3, 5.7.1 of the.2014 edition), changed the "Spatial resolution" test
method (see 6.5.3, 6.7.1 of the.2014 edition);
j) Changed the expression of the "modulation transfer function" spatial frequency (see 5.5.4, 5.7.2 of the.2014 edition), changed the "modulation transfer function"
function" refers to the version of the file (see 6.5.4, 6.7.2 of the.2014 edition);
k) Changed the expression of "quantum detection efficiency" (see 5.5.5, 5.7.3 of the.2014 edition), changed the reference to "quantum detection efficiency"
The version and recording method of the document (see 6.5.5, 6.7.3 of the.2014 edition);
l) Added "image uniformity" (see 5.5.6), added "image uniformity" test method (see 6.5.6);
m) Changed the judgment standard of "afterimage" and changed it to a quantitative standard (see 5.5.7, 5.7.4 of the.2014 edition), changed the measurement of "afterimage"
test method (see 6.5.7, 6.7.4 of the.2014 edition);
n) Changed the serial number of "artifact" (see 5.5.8, 5.7.5 of.2014 edition), changed the test method of "artifact" (see 6.5.8,.2014 edition
6.7.5);
o) Changed the "falling" judgment requirements, added intermediate and final inspection items (see 5.6.1, 5.8.1 of the.2014 edition), changed
"Falling" test tools (see 6.6.1, 6.8.1 of the.2014 edition);
p) Changed the load requirements for "bearing", increased the requirements for partial loads (see 5.6.2, 5.8.2 of the.2014 edition), changed "bearing"
The test method of "loading" was added, and the test method of partial loading was added (see 6.6.2, 6.8.2 of the.2014 edition);
q) Changed the requirements for "communication" and added requirements related to network security (see 5.7, 5.9 of the.2014 edition);
r) The requirements for "appearance" were changed, and the requirements for appearance and structure were added (see 5.8, 5.10 of the.2014 edition);
s) Changed the serial number of "test environment", changed the version of referenced documents and the serial number of intermediate inspection items (see 5.9 and 6.9,.2014
version 5.11 and 6.11);
t) Deleted "Security" (see 5.12 and 6.12 of the.2014 edition);
u) Deleted "Inspection Rules", "Identification, Labeling, Instructions for Use", "Packaging, Transportation, Storage" (see Chapter 7, Section.2014).
Chapter 8, Chapter 9).
Please note that some content of this document may be patented. The issuing agency of this document assumes no responsibility for identifying patents.
This document is proposed by the State Drug Administration.
This document is approved by the National Medical Electrical Equipment Standardization Technical Committee Medical X-ray Equipment and Appliances Sub-Technical Committee (SAC/TC10/
SC1) focal point.
This document is drafted by. Liaoning Provincial Medical Device Inspection and Testing Institute, Shanghai Yirui Optoelectronics Technology Co., Ltd., Derunte Medical Branch
Technology (Wuhan) Co., Ltd., Shanghai Food and Drug Administration Certification and Evaluation Center.
The main drafters of this document. Sun Zhiyong, Pan Wei, Wang Tongle, Zhu Yingfeng, Xu Xiaobin, Sun Jiawei, Wang Xiaotong, He Shanshan.
The previous versions of the documents replaced by this document are as follows.
---YY/T 0933-2014.
Medical General Photography Digital X-ray Image Detector
1 Scope
This document specifies the terms and definitions, classifications and groups of medical general photography digital X-ray image detectors (hereinafter referred to as detectors).
Completion, requirements, test methods.
This document applies to detectors with a single exposure imaging function, including but not limited to amorphous silicon detectors, amorphous selenium detectors, CCDs
detector, CMOS detector, etc.
This document does not apply to detectors for mammography and detectors for dental photography.
2 Normative references
The contents of the following documents constitute essential provisions of this document through normative references in the text. Among them, dated citations
documents, only the version corresponding to that date applies to this document; for undated references, the latest edition (including all amendments) applies to
this document.
GB/T 10149 Terms and Symbols of Medical X-ray Equipment
YY/T 0063 Focal characteristics of medical diagnostic X-ray tube assemblies for medical electrical equipment
YY/T 0291 Environmental requirements and test methods for medical X-ray equipment
YY/T 0481-2016 Radiation conditions for the determination of characteristics of medical diagnostic X-ray equipment
YY/T 0590.1-2018 Characteristics of digital X-ray imaging devices for medical electrical equipment Part 1-1.Quantum detection efficiency
Determination of detectors for general photography
YY/T 0741 Special technical conditions for digital photographic X-ray machines
3 Terms and Definitions
GB/T 10149, YY/T 0063, YY/T 0481-2016, YY/T 0590.1-2018 and YY/T 0741 as defined and below
Listed terms and definitions apply to this document.
3.1
calibration
The detector performs some processing necessary to make the image meet the needs of the evaluation, such as background correction, gain correction, and bad pixels
correction, etc., these processes are linear and independent of the image.
3.2
central axis
A line perpendicular to the plane of incidence and passing through the center of the field of incidence.
3.3
brightfield imagebrightimage
Raw X-ray exposure image without background, gain, and bad pixel corrections.
3.4
darkfield image darkimage
Raw unexposed image without background, gain, and bad pixel corrections.
Get Quotation: Click YY/T 0933-2022 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0933-2022
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0933-2022: Digital medical X-ray image detector used in general radiography
YY/T 0933-2022
Digital medical X-ray image detector used in general radiography
ICS 11.040.50
CCSC43
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0933-2014
Medical General Photography Digital X-ray Image Detector
Published on 2022-05-18
2023-06-01 Implementation
Released by the State Drug Administration
directory
Preface I
1 Scope 1
2 Normative references 1
3 Terms and Definitions 1
4 Classification and composition 2
5 Requirements 2
6 Test method 4
Appendix A (Normative) Test Layout 8
Appendix B (Informative) Test Device 10
foreword
This document is in accordance with the provisions of GB/T 1.1-2020 "Guidelines for Standardization Work Part 1.Structure and Drafting Rules of Standardization Documents"
drafted.
This document replaces YY/T 0933-2014 "Digital X-ray Image Detectors for Medical General Photography", this document and YY/T 0933-
Compared with.2014, the main technical changes are as follows.
a) Deleted the term and definition of "dose linear range" (see 3.1 of the.2014 edition);
b) Added five terms and definitions of "calibration", "central axis", "bright field image", "dark field image" and "noise equivalent dose" (see 3.1,
3.2, 3.3, 3.4 and 3.5);
c) Changed the definition of "linear dynamic range" (see 3.6, 3.2 of the.2014 edition);
d) Changed the description of "Classification", divided according to structural characteristics and data transmission (see 4.1, 4.1 of the.2014 edition);
e) Deleted "5.5 Dose Linear Range" (see 5.5 of the.2014 edition);
f) Changed the serial number of "image quality" (see 5.5, 5.7 of the.2014 edition);
g) Added "Noise Equivalent Dose" (see 5.5.1) and "Noise Equivalent Dose" test methods (see 6.5.1);
h) Changed the "linear dynamic range" (see 5.5.2, 5.6 of the.2014 edition), and added a criterion to express the degree of linearity of the gray value and dose
Fixed coefficient R2 (that is, the square value of the correlation coefficient r) (see 5.5.2), changed the "linear dynamic range" test method (see 6.5.2,
6.5 and 6.6 of the.2014 edition);
i) Changed "Line pair resolution" to "Spatial resolution" (see 5.5.3, 5.7.1 of the.2014 edition), changed the "Spatial resolution" test
method (see 6.5.3, 6.7.1 of the.2014 edition);
j) Changed the expression of the "modulation transfer function" spatial frequency (see 5.5.4, 5.7.2 of the.2014 edition), changed the "modulation transfer function"
function" refers to the version of the file (see 6.5.4, 6.7.2 of the.2014 edition);
k) Changed the expression of "quantum detection efficiency" (see 5.5.5, 5.7.3 of the.2014 edition), changed the reference to "quantum detection efficiency"
The version and recording method of the document (see 6.5.5, 6.7.3 of the.2014 edition);
l) Added "image uniformity" (see 5.5.6), added "image uniformity" test method (see 6.5.6);
m) Changed the judgment standard of "afterimage" and changed it to a quantitative standard (see 5.5.7, 5.7.4 of the.2014 edition), changed the measurement of "afterimage"
test method (see 6.5.7, 6.7.4 of the.2014 edition);
n) Changed the serial number of "artifact" (see 5.5.8, 5.7.5 of.2014 edition), changed the test method of "artifact" (see 6.5.8,.2014 edition
6.7.5);
o) Changed the "falling" judgment requirements, added intermediate and final inspection items (see 5.6.1, 5.8.1 of the.2014 edition), changed
"Falling" test tools (see 6.6.1, 6.8.1 of the.2014 edition);
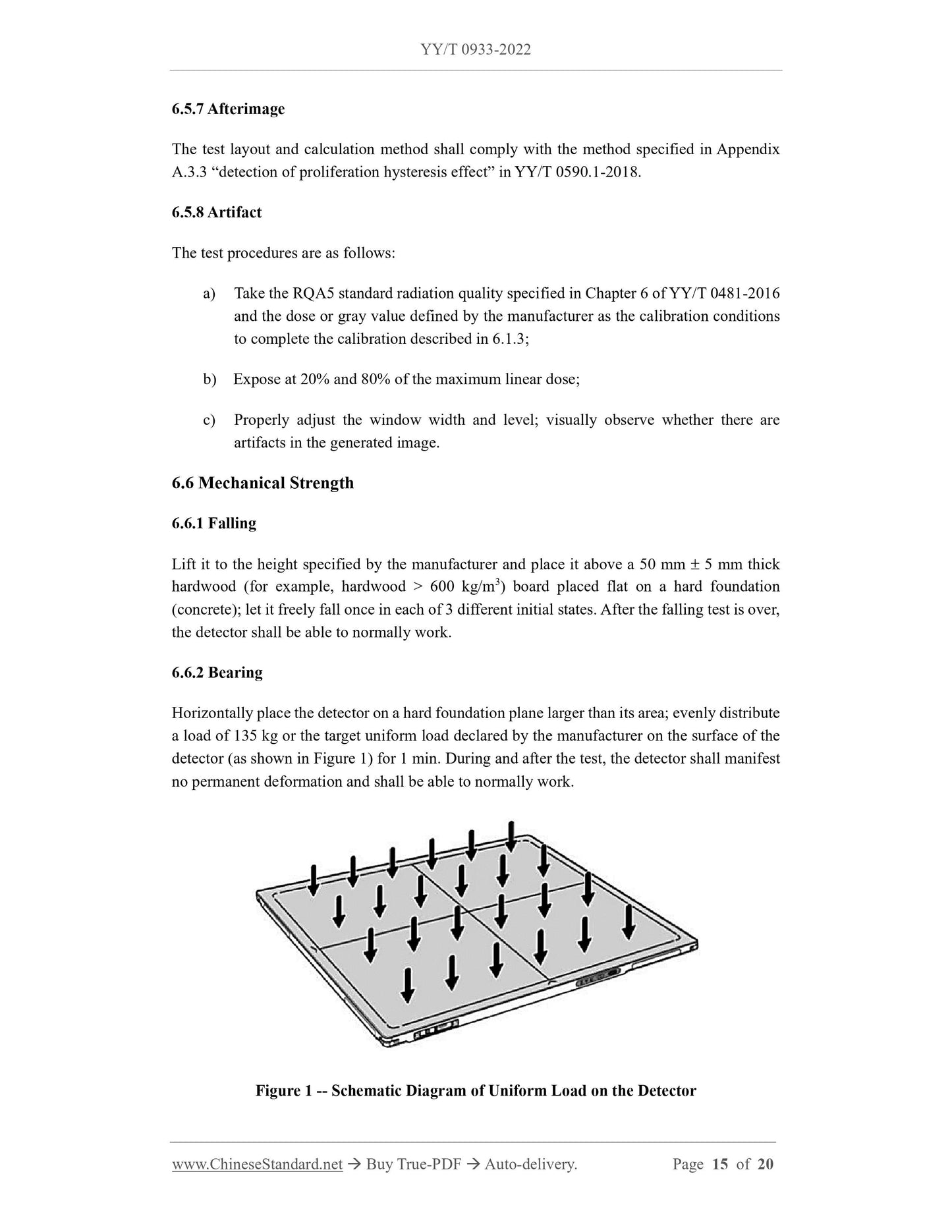
p) Changed the load requirements for "bearing", increased the requirements for partial loads (see 5.6.2, 5.8.2 of the.2014 edition), changed "bearing"
The test method of "loading" was added, and the test method of partial loading was added (see 6.6.2, 6.8.2 of the.2014 edition);
q) Changed the requirements for "communication" and added requirements related to network security (see 5.7, 5.9 of the.2014 edition);
r) The requirements for "appearance" were changed, and the requirements for appearance and structure were added (see 5.8, 5.10 of the.2014 edition);
s) Changed the serial number of "test environment", changed the version of referenced documents and the serial number of intermediate inspection items (see 5.9 and 6.9,.2014
version 5.11 and 6.11);
t) Deleted "Security" (see 5.12 and 6.12 of the.2014 edition);
u) Deleted "Inspection Rules", "Identification, Labeling, Instructions for Use", "Packaging, Transportation, Storage" (see Chapter 7, Section.2014).
Chapter 8, Chapter 9).
Please note that some content of this document may be patented. The issuing agency of this document assumes no responsibility for identifying patents.
This document is proposed by the State Drug Administration.
This document is approved by the National Medical Electrical Equipment Standardization Technical Committee Medical X-ray Equipment and Appliances Sub-Technical Committee (SAC/TC10/
SC1) focal point.
This document is drafted by. Liaoning Provincial Medical Device Inspection and Testing Institute, Shanghai Yirui Optoelectronics Technology Co., Ltd., Derunte Medical Branch
Technology (Wuhan) Co., Ltd., Shanghai Food and Drug Administration Certification and Evaluation Center.
The main drafters of this document. Sun Zhiyong, Pan Wei, Wang Tongle, Zhu Yingfeng, Xu Xiaobin, Sun Jiawei, Wang Xiaotong, He Shanshan.
The previous versions of the documents replaced by this document are as follows.
---YY/T 0933-2014.
Medical General Photography Digital X-ray Image Detector
1 Scope
This document specifies the terms and definitions, classifications and groups of medical general photography digital X-ray image detectors (hereinafter referred to as detectors).
Completion, requirements, test methods.
This document applies to detectors with a single exposure imaging function, including but not limited to amorphous silicon detectors, amorphous selenium detectors, CCDs
detector, CMOS detector, etc.
This document does not apply to detectors for mammography and detectors for dental photography.
2 Normative references
The contents of the following documents constitute essential provisions of this document through normative references in the text. Among them, dated citations
documents, only the version corresponding to that date applies to this document; for undated references, the latest edition (including all amendments) applies to
this document.
GB/T 10149 Terms and Symbols of Medical X-ray Equipment
YY/T 0063 Focal characteristics of medical diagnostic X-ray tube assemblies for medical electrical equipment
YY/T 0291 Environmental requirements and test methods for medical X-ray equipment
YY/T 0481-2016 Radiation conditions for the determination of characteristics of medical diagnostic X-ray equipment
YY/T 0590.1-2018 Characteristics of digital X-ray imaging devices for medical electrical equipment Part 1-1.Quantum detection efficiency
Determination of detectors for general photography
YY/T 0741 Special technical conditions for digital photographic X-ray machines
3 Terms and Definitions
GB/T 10149, YY/T 0063, YY/T 0481-2016, YY/T 0590.1-2018 and YY/T 0741 as defined and below
Listed terms and definitions apply to this document.
3.1
calibration
The detector performs some processing necessary to make the image meet the needs of the evaluation, such as background correction, gain correction, and bad pixels
correction, etc., these processes are linear and independent of the image.
3.2
central axis
A line perpendicular to the plane of incidence and passing through the center of the field of incidence.
3.3
brightfield imagebrightimage
Raw X-ray exposure image without background, gain, and bad pixel corrections.
3.4
darkfield image darkimage
Raw unexposed image without background, gain, and bad pixel corrections.
Share