1
/
of
7
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0980.4-2016 English PDF (YYT0980.4-2016)
YY/T 0980.4-2016 English PDF (YYT0980.4-2016)
Regular price
$140.00 USD
Regular price
Sale price
$140.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0980.4-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0980.4-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0980.4-2016: Biopsy needles for single use - Part 4: Mechanical complete type
YY/T 0980.4-2016
Biopsy needles for single use.Part 4. Mechanical complete type
ICS 11.040.20
C31
People 's Republic of China Pharmaceutical Industry Standard
One-time use of biopsy needle
Part 4. Maneuvering
Part 4. Mechanicalcompletetype
2016-07-29 released
2017-06-01 Implementation
State Food and Drug Administration issued
Preface
YY/T 0980 "One-time use of biopsy needle" series of standards are divided into the following parts.
- Part 1. General requirements;
- Part 2. Manual;
- Part 3. Motorized assembly;
- Part 4. Maneuvering.
This section is part 3 of the YY/T 0980 "Disposable biopsy needle".
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuer of this document does not assume responsibility for the identification of these patents.
This section is proposed by the State Food and Drug Administration.
This part of the National Medical Syringe (needle) Standardization Technical Committee (SAC/TC95) centralized.
This part of the drafting unit. Zhejiang Volt Medical Devices Co., Ltd., Shanghai Medical Devices Testing Institute, Shanghai Esso Medical Plastics
Co., Ltd.
This part of the main drafters. Su Weidong, Wang Zewei, Cao Xianming.
introduction
This part of YY/T 0980 specifies the requirements for a one-time use of a motorized biopsy needle.
This part does not provide sampling performance and ultrasonic development requirements and methods, because there is no suitable test method and simulation test
material.
In view of the structure of the mobile biopsy needle and the location of puncture, depth, state, texture, methods are different, this part is not on the wear
Sting is made. But the manufacturers are encouraged to combine the specific structure and use of the motorized biopsy needle produced by each manufacturer.
Corresponding puncture force.
In view of the motorized biopsy needle in the clinical use of the existence of the trigger device accidental firing phenomenon, and there is no suitable trigger device
This part of the test method is not provided, but the manufacturers are encouraged to verify the corresponding trigger device data and test
Test method.
One-time use of biopsy needle
Part 4. Maneuvering
1 Scope
YY/T 0980 this part of the provisions of a one-time use of biopsy needle --- mobile one (hereinafter referred to as "biopsy needle") requirements.
This part applies to disposable needle body and mechanical power unit in the manufacture of that is combined into one, the use of mechanical power
The device performs an automatic or semi-automatic cutting operation to collect a one-time use of a manual tissue biopsy needle.
2 normative reference documents
The following documents are indispensable for the application of this document. For dated references, the only dated edition applies to this article
Pieces. For undated references, the latest edition (including all modifications) applies to this document.
GB/T 1220 stainless steel bar
GB/T 2965 Titanium and Titanium Alloy Bar
Disposable biopsies for disposable use - Part 1. General requirements for YY/T 0980.1-2016
3 structure type
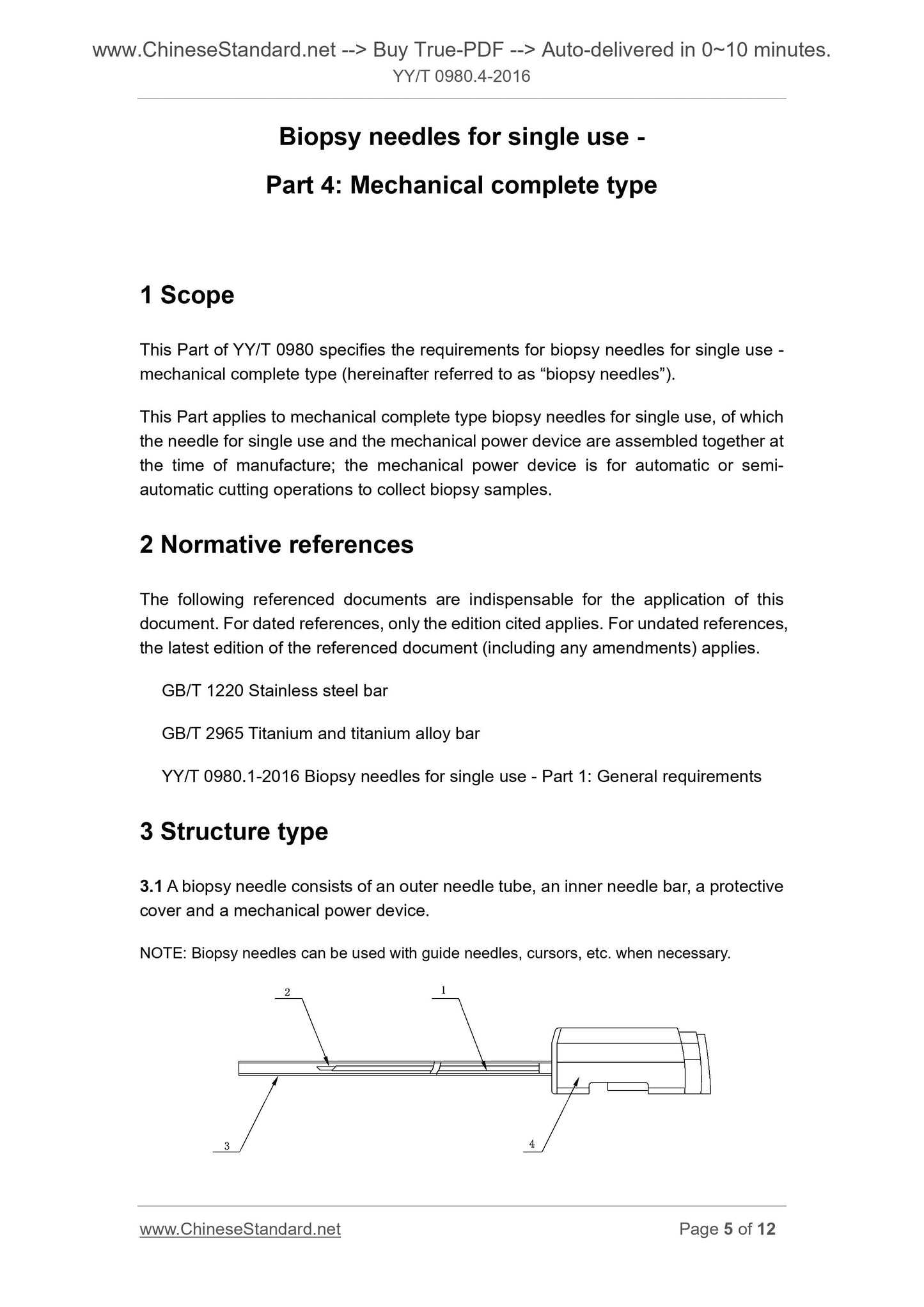
3.1 biopsy needle from the outer needle, the needle bar, sheath, mechanical power device components, as shown in Figure 1.
Note. biopsy needle with the need to guide the needle, such as the use of cursor.
Description.
1 --- outer needle;
2 --- inner needle bar;
3 --- Sheath;
4 - mechanical power unit.
Figure 1 Schematic diagram of a typical structure of a motorized biopsy needle
3.2 biopsy needle sampling pattern is divided into slot cutting and tube cutting two types, as shown in Figure 2.
A) Trough cutting sampling type
Description.
1 --- inner needle bar;
2 --- outside the needle.
A Outside the needle tip.
B inner needle bar slope root.
C End of needle bar sampling edge.
B) Tubular cutting sampling type
Description.
1 --- inner needle bar;
2 --- sampling needle;
3 --- outer needle.
A Sampling needle tip at the top.
B inner needle bar slope root.
Figure 2 biopsy needle cutting sampling type typical structure diagram
4 material
4.1 The needle of the biopsy needle shall comply with the requirements of 5.2 in YY/T 0980.1-2016.
4.2 needle needle material should be used GB/T 1220 specified in the austenitic stainless steel (such as. 06Cr19Ni10,022
Cr19Ni10, 06Cr18Ni11Ti), nickel-chromium and nickel-titanium alloy materials or GB/T 2965 in the titanium and titanium alloy materials
production.
5 requirements and test methods
5.1 General
In addition to the YQ/T 0980.1-2016 5.3 ~ 5.13 in accordance with the relevant requirements, but also should meet the following requirements.
Note. The requirements of 5.11, 5.12, 5.13 above are limited to the biopsy needle part.
5.2 Cutting the sampling type
5.2.1 Slotting of the needle The needle tip of the needle should not extend beyond the root of the inner bevel edge of the needle, and the edge of the front end of the inner needle bar
Can not exceed the top of the outer needle tip.
5.2.2 Tubular cutting biopsy needle The tip of the needle of the sampling needle should not extend beyond the root of the bevel edge of the needle bar in the puncture state;
After the hair, the cutting bar should be able to automatically reach the cutting position, as shown in Figure 3.
Figure 3 Typical pattern of tube cutting
5.3 Needle bar connection fastness
The needle bar of the biopsy needle should be firmly connected with the mechanical power unit and the axial direction shown in Table 1 is applied to the inner needle bar and the mechanical power unit
Static tension, for 10s, the two shall not be separated.
Table 1 Connection fastness
Inner needle outer diameter
Mm
pull
< 0.5 10
0.5 to 1.0 15
> 1.0 20
5.4 scale logo
Biopsy needle outside the needle, such as marked with a puncture depth prompted the scale line (shown in Figure 4), the scale line should be formed in the shape of the needle ring, clear
Discernible In the puncture state to be detected by a universal gage, the inner needle tip to the front end of the first scale line and any two adjacent scale
The distance between the leading edge of the line should be 10 mm ± 1.5 mm and the distance from the tip to the front edge of the last scale should be
(N × 10) mm ± 2.0 mm.
Note. If you want to distinguish between 50mm, 100mm, 150mm,.200mm and other depth position, should be unified with the first line of the front line mark prevail.
The unit is in millimeters
Figure 4 biopsy needle outside the needle typical scale line diagram
5.5 Mechanical power unit
5.5.1 Trigger device
When pulling the outer needle and the inner needle bar to the limited position, and the trigger test for 10 consecutive times, should be smooth, no jamming phenomenon.
5.5.2 safety lock
If the biopsy needle is with a safety catch, the trigger trigger should not be activated when the safety lock is in the locked position.
5.5.3 Sampling length positioning device
If the biopsy needle is equipped with a sampling length positioning device, the sampling length should be marked at the corresponding position of the mechanical power unit. Measured with a common gauge
The distance between the moving distance of the outside needle and the size of the sample should not exceed 5%.
5.6 Sheath
The needle of the biopsy needle should have a sheath, and the sheath should not fall off naturally and should be easily removed when used.
6 Packing and logo
The biopsy needle packaging and marking shall comply with the requirements of Chapters 6 and 7 of YY/T 0980.1-2016.
7 User's Manual
The instruction manual should have at least the following information.
A) product name;
B) Product model description;
C) product performance, the main structure;
D) the scope of application;
E) use methods and precautions;
F) storage conditions;
G) other instructions and necessary warnings.
8 transport and storage
8.1 biopsy needle in the transport, storage process should be to prevent stress, sun and rain and snow leaching.
8.2 biopsy needles should be stored in a non-corrosive gas, well ventilated and clean environment.
9 type inspection rules
See Appendix A for the type inspection rules.
Appendix A
(Informative)
Type inspection rules
A.1 Test items
The inspection item is the requirement of Chapter 5.
A.2 Sampling quantity
Physical performance random sampling of 10, chemical and biological performance testing on-demand extraction.
A.3 determine the rules
All inspection items are qualified, through the type of inspection. If the chemical and biological properties are all qualified, the physical performance is unqualified
, It is allowed to double the sample again and the nonconformity item is re-examined. Re-test is still unqualified, then the type of inspection does not pass.
references
[1] GB 15811-2001 disposable sterile injection needle
One - time use of needle for anesthesia [2] YY 0321.2-2009
[3] YY/T 0313-2014 Medical polymer packaging and manufacturers to provide information requirements
[4] YY/T 0466.1-2009 Symbols for medical devices for labeling, labeling and providing information on medical devices - Part 1.
General requirements
Get Quotation: Click YY/T 0980.4-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0980.4-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0980.4-2016: Biopsy needles for single use - Part 4: Mechanical complete type
YY/T 0980.4-2016
Biopsy needles for single use.Part 4. Mechanical complete type
ICS 11.040.20
C31
People 's Republic of China Pharmaceutical Industry Standard
One-time use of biopsy needle
Part 4. Maneuvering
Part 4. Mechanicalcompletetype
2016-07-29 released
2017-06-01 Implementation
State Food and Drug Administration issued
Preface
YY/T 0980 "One-time use of biopsy needle" series of standards are divided into the following parts.
- Part 1. General requirements;
- Part 2. Manual;
- Part 3. Motorized assembly;
- Part 4. Maneuvering.
This section is part 3 of the YY/T 0980 "Disposable biopsy needle".
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuer of this document does not assume responsibility for the identification of these patents.
This section is proposed by the State Food and Drug Administration.
This part of the National Medical Syringe (needle) Standardization Technical Committee (SAC/TC95) centralized.
This part of the drafting unit. Zhejiang Volt Medical Devices Co., Ltd., Shanghai Medical Devices Testing Institute, Shanghai Esso Medical Plastics
Co., Ltd.
This part of the main drafters. Su Weidong, Wang Zewei, Cao Xianming.
introduction
This part of YY/T 0980 specifies the requirements for a one-time use of a motorized biopsy needle.
This part does not provide sampling performance and ultrasonic development requirements and methods, because there is no suitable test method and simulation test
material.
In view of the structure of the mobile biopsy needle and the location of puncture, depth, state, texture, methods are different, this part is not on the wear
Sting is made. But the manufacturers are encouraged to combine the specific structure and use of the motorized biopsy needle produced by each manufacturer.
Corresponding puncture force.
In view of the motorized biopsy needle in the clinical use of the existence of the trigger device accidental firing phenomenon, and there is no suitable trigger device
This part of the test method is not provided, but the manufacturers are encouraged to verify the corresponding trigger device data and test
Test method.
One-time use of biopsy needle
Part 4. Maneuvering
1 Scope
YY/T 0980 this part of the provisions of a one-time use of biopsy needle --- mobile one (hereinafter referred to as "biopsy needle") requirements.
This part applies to disposable needle body and mechanical power unit in the manufacture of that is combined into one, the use of mechanical power
The device performs an automatic or semi-automatic cutting operation to collect a one-time use of a manual tissue biopsy needle.
2 normative reference documents
The following documents are indispensable for the application of this document. For dated references, the only dated edition applies to this article
Pieces. For undated references, the latest edition (including all modifications) applies to this document.
GB/T 1220 stainless steel bar
GB/T 2965 Titanium and Titanium Alloy Bar
Disposable biopsies for disposable use - Part 1. General requirements for YY/T 0980.1-2016
3 structure type
3.1 biopsy needle from the outer needle, the needle bar, sheath, mechanical power device components, as shown in Figure 1.
Note. biopsy needle with the need to guide the needle, such as the use of cursor.
Description.
1 --- outer needle;
2 --- inner needle bar;
3 --- Sheath;
4 - mechanical power unit.
Figure 1 Schematic diagram of a typical structure of a motorized biopsy needle
3.2 biopsy needle sampling pattern is divided into slot cutting and tube cutting two types, as shown in Figure 2.
A) Trough cutting sampling type
Description.
1 --- inner needle bar;
2 --- outside the needle.
A Outside the needle tip.
B inner needle bar slope root.
C End of needle bar sampling edge.
B) Tubular cutting sampling type
Description.
1 --- inner needle bar;
2 --- sampling needle;
3 --- outer needle.
A Sampling needle tip at the top.
B inner needle bar slope root.
Figure 2 biopsy needle cutting sampling type typical structure diagram
4 material
4.1 The needle of the biopsy needle shall comply with the requirements of 5.2 in YY/T 0980.1-2016.
4.2 needle needle material should be used GB/T 1220 specified in the austenitic stainless steel (such as. 06Cr19Ni10,022
Cr19Ni10, 06Cr18Ni11Ti), nickel-chromium and nickel-titanium alloy materials or GB/T 2965 in the titanium and titanium alloy materials
production.
5 requirements and test methods
5.1 General
In addition to the YQ/T 0980.1-2016 5.3 ~ 5.13 in accordance with the relevant requirements, but also should meet the following requirements.
Note. The requirements of 5.11, 5.12, 5.13 above are limited to the biopsy needle part.
5.2 Cutting the sampling type
5.2.1 Slotting of the needle The needle tip of the needle should not extend beyond the root of the inner bevel edge of the needle, and the edge of the front end of the inner needle bar
Can not exceed the top of the outer needle tip.
5.2.2 Tubular cutting biopsy needle The tip of the needle of the sampling needle should not extend beyond the root of the bevel edge of the needle bar in the puncture state;
After the hair, the cutting bar should be able to automatically reach the cutting position, as shown in Figure 3.
Figure 3 Typical pattern of tube cutting
5.3 Needle bar connection fastness
The needle bar of the biopsy needle should be firmly connected with the mechanical power unit and the axial direction shown in Table 1 is applied to the inner needle bar and the mechanical power unit
Static tension, for 10s, the two shall not be separated.
Table 1 Connection fastness
Inner needle outer diameter
Mm
pull
< 0.5 10
0.5 to 1.0 15
> 1.0 20
5.4 scale logo
Biopsy needle outside the needle, such as marked with a puncture depth prompted the scale line (shown in Figure 4), the scale line should be formed in the shape of the needle ring, clear
Discernible In the puncture state to be detected by a universal gage, the inner needle tip to the front end of the first scale line and any two adjacent scale
The distance between the leading edge of the line should be 10 mm ± 1.5 mm and the distance from the tip to the front edge of the last scale should be
(N × 10) mm ± 2.0 mm.
Note. If you want to distinguish between 50mm, 100mm, 150mm,.200mm and other depth position, should be unified with the first line of the front line mark prevail.
The unit is in millimeters
Figure 4 biopsy needle outside the needle typical scale line diagram
5.5 Mechanical power unit
5.5.1 Trigger device
When pulling the outer needle and the inner needle bar to the limited position, and the trigger test for 10 consecutive times, should be smooth, no jamming phenomenon.
5.5.2 safety lock
If the biopsy needle is with a safety catch, the trigger trigger should not be activated when the safety lock is in the locked position.
5.5.3 Sampling length positioning device
If the biopsy needle is equipped with a sampling length positioning device, the sampling length should be marked at the corresponding position of the mechanical power unit. Measured with a common gauge
The distance between the moving distance of the outside needle and the size of the sample should not exceed 5%.
5.6 Sheath
The needle of the biopsy needle should have a sheath, and the sheath should not fall off naturally and should be easily removed when used.
6 Packing and logo
The biopsy needle packaging and marking shall comply with the requirements of Chapters 6 and 7 of YY/T 0980.1-2016.
7 User's Manual
The instruction manual should have at least the following information.
A) product name;
B) Product model description;
C) product performance, the main structure;
D) the scope of application;
E) use methods and precautions;
F) storage conditions;
G) other instructions and necessary warnings.
8 transport and storage
8.1 biopsy needle in the transport, storage process should be to prevent stress, sun and rain and snow leaching.
8.2 biopsy needles should be stored in a non-corrosive gas, well ventilated and clean environment.
9 type inspection rules
See Appendix A for the type inspection rules.
Appendix A
(Informative)
Type inspection rules
A.1 Test items
The inspection item is the requirement of Chapter 5.
A.2 Sampling quantity
Physical performance random sampling of 10, chemical and biological performance testing on-demand extraction.
A.3 determine the rules
All inspection items are qualified, through the type of inspection. If the chemical and biological properties are all qualified, the physical performance is unqualified
, It is allowed to double the sample again and the nonconformity item is re-examined. Re-test is still unqualified, then the type of inspection does not pass.
references
[1] GB 15811-2001 disposable sterile injection needle
One - time use of needle for anesthesia [2] YY 0321.2-2009
[3] YY/T 0313-2014 Medical polymer packaging and manufacturers to provide information requirements
[4] YY/T 0466.1-2009 Symbols for medical devices for labeling, labeling and providing information on medical devices - Part 1.
General requirements
Share