1
/
of
11
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0988.2-2016 English PDF (YY/T0988.2-2016)
YY/T 0988.2-2016 English PDF (YY/T0988.2-2016)
Regular price
$140.00 USD
Regular price
Sale price
$140.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0988.2-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0988.2-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0988.2-2016: Coatings of surgical implants - Part 2: Titanium and titanium-6 aluminum-4 vanadium alloy powders
YY/T 0988.2-2016
Coatings of surgical implants - Part 2. Titanium and titanium-6 aluminum-4 vanadium alloy powders
ICS 11.040.40
C35
People's Republic of China Pharmaceutical Industry Standard
Surgical Implant Coatings
Part 2. titanium and titanium - 6 aluminum - 4 vanadium alloy powder
Coatingsofsurgicalimplants-Part 2. Titaniumandtitanium-6aluminum-4
2016-03-23 release
2017-01-01 Implementation
State Food and Drug Administration issued
Directory
Preface III
1 range 1
2 normative reference document 1
3 meaning and application 1
4 manufacturing method 1
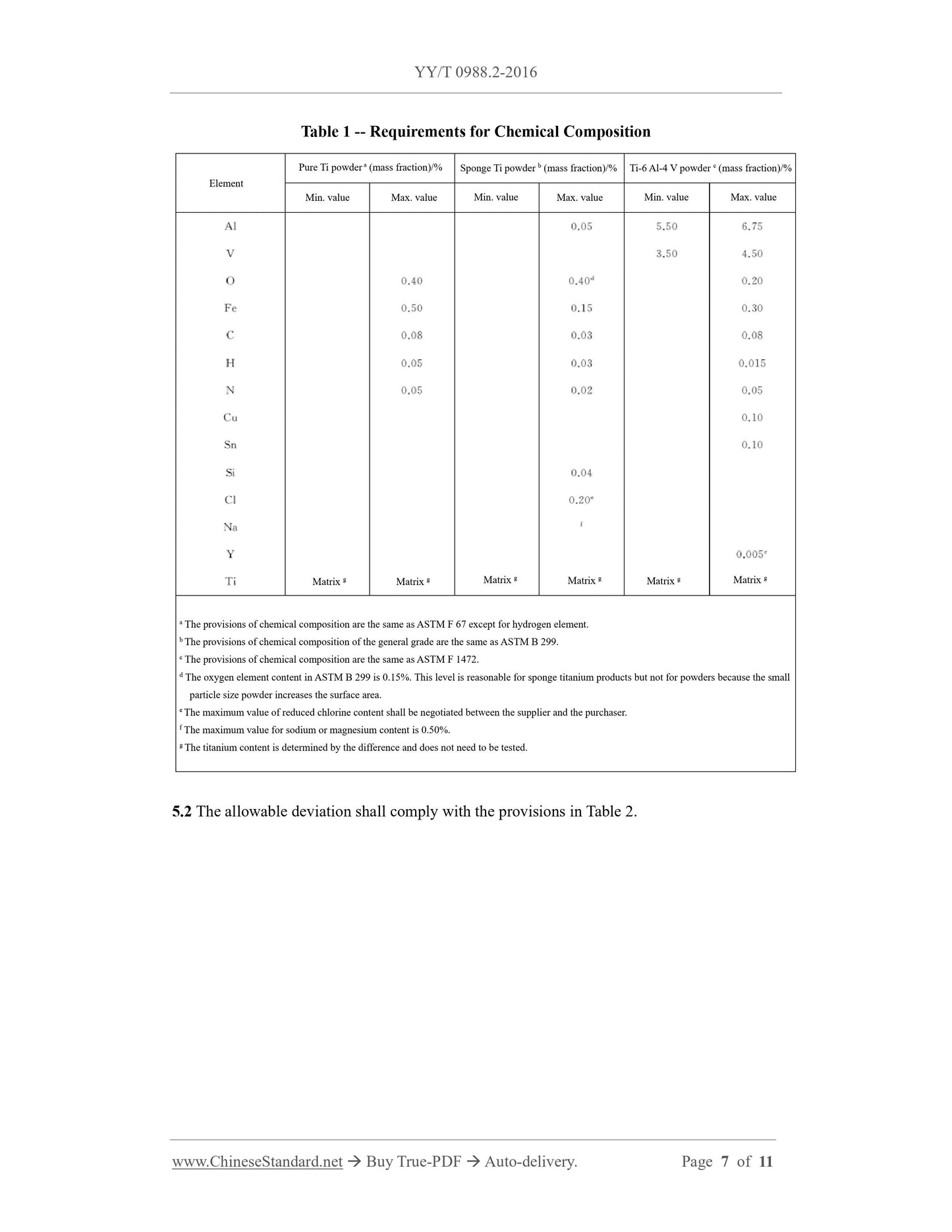
5 Chemical requirements 2
6 particle size 3
7 cleanliness 3
Appendix A (informative) Basic principles 4
Appendix B (informative) Biocompatibility 5
Preface
YY/T 0988 "Surgical Implant Coating" is divided into the following sections.
- Part 1. cobalt - 28 chromium - 6 molybdenum powder;
- Part 2. Titanium and titanium - 6 - 4 - vanadium alloy powders;
- Part 3 to Part 10. (reserved);
- Part 11. Test methods for tensile test of calcium phosphate coating and metal coating;
Part 12. Test methods for calcium phosphate coating and metal coating shear test;
- Part 13. Test methods for shear and bending fatigue of calcium phosphate, metal and calcium phosphate/metal composite coatings;
- Part 14. Method for evaluation of porous coating bodies;
Part 15. Test method for abrasion resistance of metallic thermal sprayed coatings.
This part is part 2 of YY/T 0988.
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
This section uses the re-drafting method for reference to ASTM F1580-2007 "Titanium and Titanium-6-Aluminum-4 Vanadium Alloy Powders for Surgical Implant Coatings
Standard specification ".
The technical differences between this section and ASTM F1580-2007 are as follows.
--- on the normative reference documents, this part has made a technical differences in the adjustment, delete 2.2,2.3, to adapt to China's technology
condition.
- Remove Chapter 5, Chapter 9, Chapter 10, Chapter 11 of ASTM F1580-2007.
Please note that some of the contents of this document may involve patents. The issuer of this document does not assume responsibility for the identification of these patents.
This section is proposed by the State Food and Drug Administration.
This section is under the jurisdiction of the National Standardization Technical Committee on Surgical Implants and Orthopedic Instruments (SAC/TC110).
This part of the drafting unit. the State Food and Drug Administration Tianjin Medical Device Quality Supervision and Inspection Center, the State Food and Drug Administration
Bureau of Medical Device Technical Review Center.
The main drafters of this part. Li Yuan, Fu Ruizhi, Dong Shuangpeng, Liu Bin, Zhang Jiazhen, Dong Wenxing.
Surgical Implant Coatings
Part 2. titanium and titanium - 6 aluminum - 4 vanadium alloy powder
1 Scope
This part of YY/T 0988 specifies the use of pure titanium powder and titanium-6 aluminum-4 vanadium alloy powder for the formation of coatings on titanium alloy implants
Claim.
The powder specified in this section may be formed by a sintering process or a thermal spraying process.
This section specifies the requirements of the powder and does not apply to the performance of the powder made of the coating.
Note. Fine titanium powder may cause spontaneous combustion, handling should be consistent with the relevant guidelines.
2 normative reference documents
The following documents are indispensable for the application of this document. For dated references, only the dated edition applies to this article
Pieces. For undated references, the latest edition (including all modifications) applies to this document.
ASTMB214 metal powder sieving standard test method (Testmethodforsieveanalysisofmetalpowders)
Standard Practice for the Sampling of ASTMB215 Metal Powders (Practicesforsamplingmetalpowders)
ASTMB299 Titanium Sponge Specification (Specificationfortitaniumsponge)
ASTME11 Specification for Wire Mesh Screening (Specificationforwireclothandsievesfortestingpurposes)
Standard Test Method for Determination of Titanium and Titanium Alloys by Atomic Emission Plasma Spectrometry with ASTME2371 (Testmethodfor
analysisoftitaniumandtitaniumaloysbyatomicemissionplasmaspectrometry
ASTM F67 surgical implants are made of pure titanium (Specification forunaloyedtitanium, forsurgicalimplant
applications)
ASTM F1472 surgical implants forged titanium-6 aluminum-4 vanadium alloy specification (Specificationforwroughttitanium-
6aluminum-4vanadiumaloyforsurgicalimplantapplications)
AMS2249 titanium and titanium alloy allowable deviation (Chemicalcheckanalysislimits, titaniumandtitaniumaloys)
AMS4998 6 aluminum-4 vanadium powder (Powder, 6Al-4V)
3 meaning and application
The coating made of metal powder has been widely used in improving the adhesion of tissue and non-cement fixed joint prosthesis. Such coating also
Can improve the adhesion of acrylic resin bone cement and prosthesis. This section specifies the specific requirements for the coating of metallic powders. Principle and health
See Annex A and Appendix B for compatibility.
4 manufacturing method
The powder may be subjected to a plasma rotary electrode method, an inert gas atomization method, a hydrogenation-dehydrogenation method or a powder capable of producing a powder satisfying the requirements of this part
Of other methods.
Get Quotation: Click YY/T 0988.2-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0988.2-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0988.2-2016: Coatings of surgical implants - Part 2: Titanium and titanium-6 aluminum-4 vanadium alloy powders
YY/T 0988.2-2016
Coatings of surgical implants - Part 2. Titanium and titanium-6 aluminum-4 vanadium alloy powders
ICS 11.040.40
C35
People's Republic of China Pharmaceutical Industry Standard
Surgical Implant Coatings
Part 2. titanium and titanium - 6 aluminum - 4 vanadium alloy powder
Coatingsofsurgicalimplants-Part 2. Titaniumandtitanium-6aluminum-4
2016-03-23 release
2017-01-01 Implementation
State Food and Drug Administration issued
Directory
Preface III
1 range 1
2 normative reference document 1
3 meaning and application 1
4 manufacturing method 1
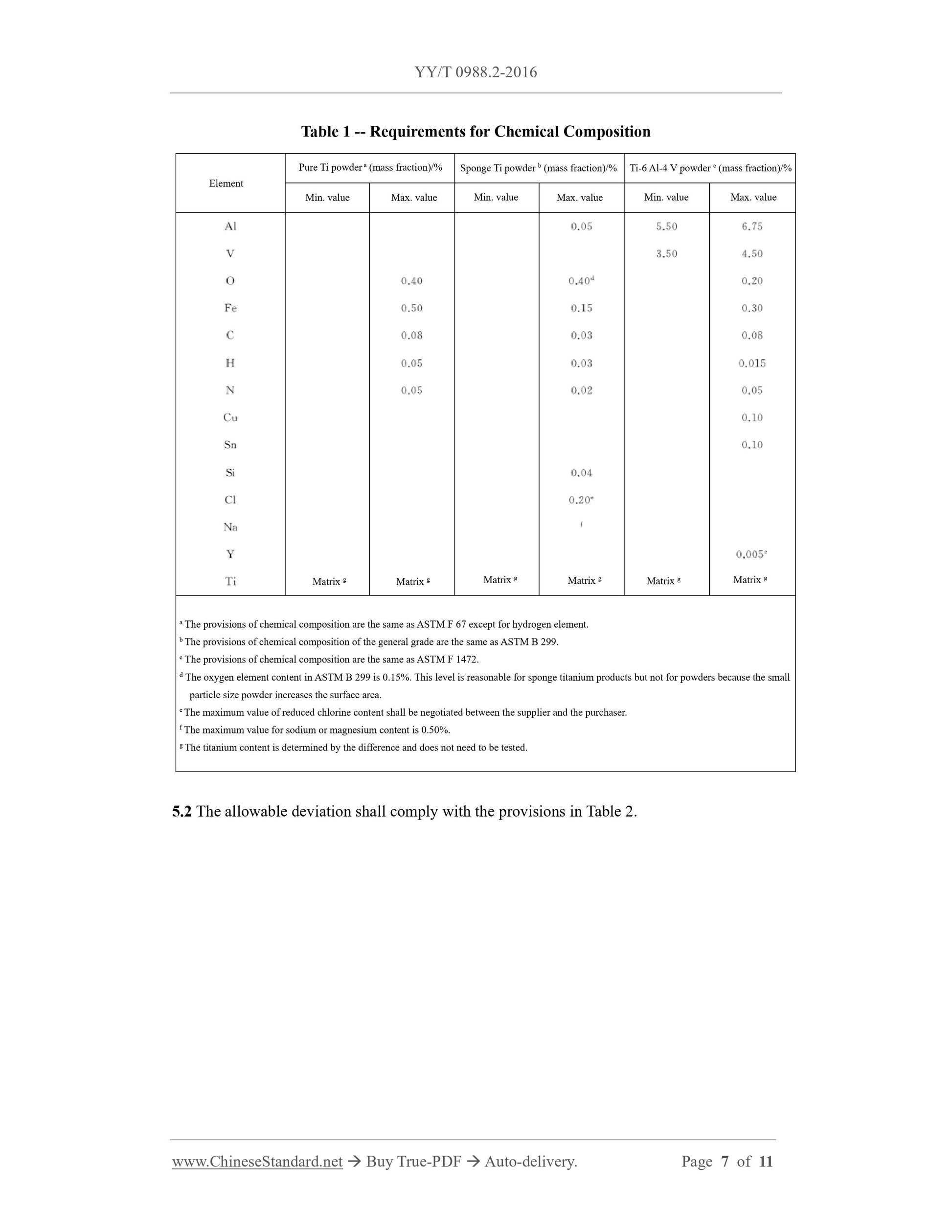
5 Chemical requirements 2
6 particle size 3
7 cleanliness 3
Appendix A (informative) Basic principles 4
Appendix B (informative) Biocompatibility 5
Preface
YY/T 0988 "Surgical Implant Coating" is divided into the following sections.
- Part 1. cobalt - 28 chromium - 6 molybdenum powder;
- Part 2. Titanium and titanium - 6 - 4 - vanadium alloy powders;
- Part 3 to Part 10. (reserved);
- Part 11. Test methods for tensile test of calcium phosphate coating and metal coating;
Part 12. Test methods for calcium phosphate coating and metal coating shear test;
- Part 13. Test methods for shear and bending fatigue of calcium phosphate, metal and calcium phosphate/metal composite coatings;
- Part 14. Method for evaluation of porous coating bodies;
Part 15. Test method for abrasion resistance of metallic thermal sprayed coatings.
This part is part 2 of YY/T 0988.
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
This section uses the re-drafting method for reference to ASTM F1580-2007 "Titanium and Titanium-6-Aluminum-4 Vanadium Alloy Powders for Surgical Implant Coatings
Standard specification ".
The technical differences between this section and ASTM F1580-2007 are as follows.
--- on the normative reference documents, this part has made a technical differences in the adjustment, delete 2.2,2.3, to adapt to China's technology
condition.
- Remove Chapter 5, Chapter 9, Chapter 10, Chapter 11 of ASTM F1580-2007.
Please note that some of the contents of this document may involve patents. The issuer of this document does not assume responsibility for the identification of these patents.
This section is proposed by the State Food and Drug Administration.
This section is under the jurisdiction of the National Standardization Technical Committee on Surgical Implants and Orthopedic Instruments (SAC/TC110).
This part of the drafting unit. the State Food and Drug Administration Tianjin Medical Device Quality Supervision and Inspection Center, the State Food and Drug Administration
Bureau of Medical Device Technical Review Center.
The main drafters of this part. Li Yuan, Fu Ruizhi, Dong Shuangpeng, Liu Bin, Zhang Jiazhen, Dong Wenxing.
Surgical Implant Coatings
Part 2. titanium and titanium - 6 aluminum - 4 vanadium alloy powder
1 Scope
This part of YY/T 0988 specifies the use of pure titanium powder and titanium-6 aluminum-4 vanadium alloy powder for the formation of coatings on titanium alloy implants
Claim.
The powder specified in this section may be formed by a sintering process or a thermal spraying process.
This section specifies the requirements of the powder and does not apply to the performance of the powder made of the coating.
Note. Fine titanium powder may cause spontaneous combustion, handling should be consistent with the relevant guidelines.
2 normative reference documents
The following documents are indispensable for the application of this document. For dated references, only the dated edition applies to this article
Pieces. For undated references, the latest edition (including all modifications) applies to this document.
ASTMB214 metal powder sieving standard test method (Testmethodforsieveanalysisofmetalpowders)
Standard Practice for the Sampling of ASTMB215 Metal Powders (Practicesforsamplingmetalpowders)
ASTMB299 Titanium Sponge Specification (Specificationfortitaniumsponge)
ASTME11 Specification for Wire Mesh Screening (Specificationforwireclothandsievesfortestingpurposes)
Standard Test Method for Determination of Titanium and Titanium Alloys by Atomic Emission Plasma Spectrometry with ASTME2371 (Testmethodfor
analysisoftitaniumandtitaniumaloysbyatomicemissionplasmaspectrometry
ASTM F67 surgical implants are made of pure titanium (Specification forunaloyedtitanium, forsurgicalimplant
applications)
ASTM F1472 surgical implants forged titanium-6 aluminum-4 vanadium alloy specification (Specificationforwroughttitanium-
6aluminum-4vanadiumaloyforsurgicalimplantapplications)
AMS2249 titanium and titanium alloy allowable deviation (Chemicalcheckanalysislimits, titaniumandtitaniumaloys)
AMS4998 6 aluminum-4 vanadium powder (Powder, 6Al-4V)
3 meaning and application
The coating made of metal powder has been widely used in improving the adhesion of tissue and non-cement fixed joint prosthesis. Such coating also
Can improve the adhesion of acrylic resin bone cement and prosthesis. This section specifies the specific requirements for the coating of metallic powders. Principle and health
See Annex A and Appendix B for compatibility.
4 manufacturing method
The powder may be subjected to a plasma rotary electrode method, an inert gas atomization method, a hydrogenation-dehydrogenation method or a powder capable of producing a powder satisfying the requirements of this part
Of other methods.
Share