1
/
of
8
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1090-2018 English PDF (YY/T1090-2018)
YY/T 1090-2018 English PDF (YY/T1090-2018)
Regular price
$100.00 USD
Regular price
Sale price
$100.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1090-2018 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1090-2018
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1090-2018: Ultrasonic physiotherapy equipment
YY/T 1090-2018
Ultrasonic physiotherapy equipment
ICS 11.040.60
C41
People's Republic of China Pharmaceutical Industry Standard
Replacing YY 1090-2009
Ultrasound therapy equipment
Published on.2018-12-20
2020-01-01 implementation
State Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY 1090-2009 "ultrasound physiotherapy equipment", compared with YY 1090-2009, except for editorial modification, the main technology
The changes are as follows.
---Modified the content of effective sound intensity (4.3, 4.2 of the.2009 edition);
--- Increased output power announcement (4.1,.2009 version 4.1.1);
--- Increased requirements for effective radiation area (4.2);
--- Increased the requirements for random files (4.7);
--- Removed the requirement to repeat with GB 9706.7-2008 (see 4.1.2, 4.1.3, 4.1.4, 4.1.5, 4.5 and 4.6 of the.2009 edition).
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Drug Administration.
This standard is under the jurisdiction of the National Medical Electrical Equipment Standardization Technical Committee Medical Ultrasound Equipment Subcommittee (SAC/TC10/SC2).
This standard was drafted. Hubei Medical Device Quality Supervision and Inspection Institute.
The main drafters of this standard. Jiang Shilin, Wang Zhiwei.
The previous versions of the standards replaced by this standard are.
---ZBC42012-1989;
---YY 91090-1999;
---YY/T 1090-2004;
---YY 1090-2009.
Ultrasound therapy equipment
1 Scope
This standard specifies the requirements, test methods and inspection rules for ultrasonic physiotherapy equipment.
This standard is applicable to continuous wave or quasi-continuous wave ultrasonic energy generated by a planar circular ultrasonic transducer in the frequency range of 0.5MHz~5MHz.
Amount of ultrasonic physiotherapy equipment (hereinafter referred to as equipment), this standard does not apply to effective sound stronger than 3W/cm2 or using focused ultrasound
device.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB 9706.1 Medical electrical equipment - Part 1. General requirements for safety
GB 9706.7 Medical electrical equipment - Part 2-5. Particular requirements for safety of physiotherapy equipment
GB/T 14710 Medical electrical equipment environmental requirements and test methods
YY/T 0750-2018 Sound field requirements and measurement methods in the frequency range of 0.5MHz~5MHz for ultrasonic physiotherapy equipment
YY/T 0865.1-2011 Ultrasonic hydrophones - Part 1. Measurement and characterization of medical ultrasound fields below 40 MHz
3 Terms and definitions
The terms and definitions defined in GB 9706.7 and YY/T 0750 apply to this document.
4 requirements
4.1 Accuracy of ultrasonic output power
The manufacturer shall publish the rated ultrasonic output power and the rated ultrasonic time maximum output power (if applicable), within its range, the power indication
The deviation of the value from the actual measured value shall not exceed ±20%.
4.2 Effective radiation area
The manufacturer shall publish the effective radiation area, and the deviation between the actual measured value and the published value shall not exceed the value published by the manufacturer.
4.3 Effective sound intensity
The manufacturer shall announce the maximum effective sound intensity at the nominal output power rating.
4.4 Acoustic operating frequency
The manufacturer shall announce the acoustic operating frequency, and the deviation between the actual measured value and the published value shall not exceed 10%.
4.5 Beam unevenness coefficient
RBN
The absolute maximum beam non-uniformity factor of all treatment heads or additional heads shall not exceed the manufacturer's published value and shall not exceed 8.0.
4.6 Appearance and structure
The appearance should be uniform in color, clean and tidy, free of scratches, cracks and other defects.
The text and logo on the panel should be legible and long-lasting.
The control and adjustment mechanism should be flexible and reliable, and the fastening parts are not loose.
4.7 Random files
The manufacturer shall publish the parameters to be published in 4.1~4.7 in the random file.
4.8 Safety requirements
The safety of the equipment shall comply with the requirements of GB 9706.1 and GB 9706.7.
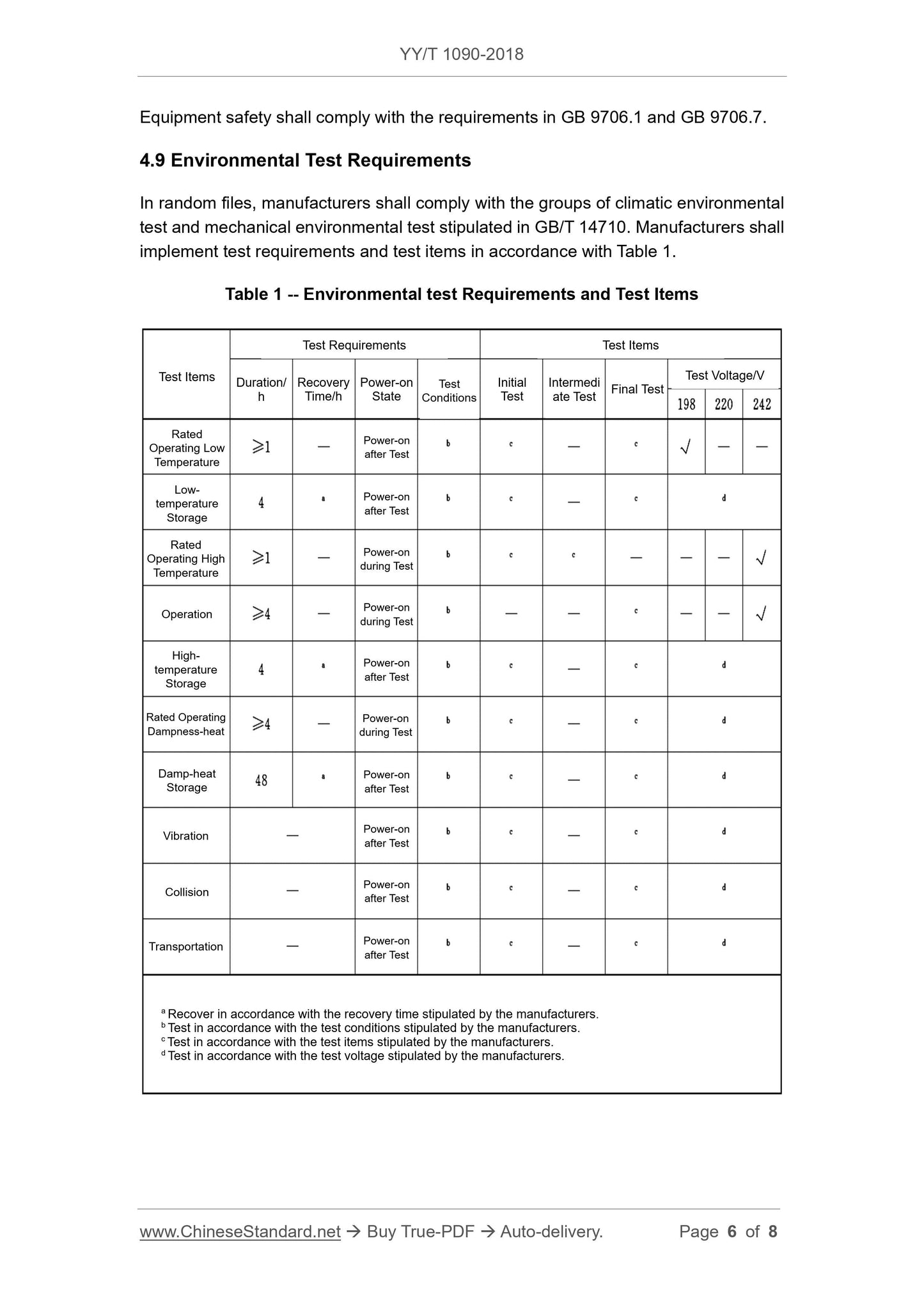
4.9 Environmental test requirements
The manufacturer shall, in the random documents, the group of climatic environmental tests and mechanical environmental tests specified in GB/T 14710, test requirements and inspections.
The test items are implemented in accordance with Table 1.
Table 1 Environmental test requirements and inspection items
a Recovers at the recovery time specified by the manufacturer.
b Test according to the test conditions specified by the manufacturer.
c Test according to the test items specified by the manufacturer.
d Test voltage test according to the manufacturer's specifications.
5 Test methods
5.1 Accuracy of ultrasonic output power
The ultrasonic output power is carried out in accordance with the test methods specified in 7.2 and 7.3 of YY/T 0750-2018.
The rated ultrasonic output power and the rated ultrasonic time maximum output power shall be measured at 90%, 100% of the rated supply voltage, respectively.
Under the condition of 110%, it is not allowed to manually adjust the equipment during the measurement, take the most unfavorable value, and check whether the measurement result meets the requirements.
Measured at 1/3, 2/3 (or nearby, or gear) of the ultrasonic output power and the rated ultrasonic output maximum output power range, respectively.
The deviation between the indicated value of power and the actual measured value.
5.2 Effective radiation area
This is carried out in accordance with the test method specified in 7.4 of YY/T 0750-2018. The deviation is calculated according to equation (1).
5.3 Effective sound intensity
The effective sound intensity is calculated from the measured values of the output power and the effective radiation area.
5.4 Acoustic operating frequency
It is carried out in accordance with the test method specified in 7.3 of YY/T 0865.1-2011 and YY/T 0750-2018. The deviation is calculated according to equation (1).
5.5 Beam non-uniformity coefficient
This is carried out in accordance with the test method specified in 7.4 of YY/T 0750-2018.
5.6 Appearance and structure
Visual inspection should meet the requirements of 4.7.
5.7 Random files
Check random documents and meet the requirements of 4.8.
5.8 Safety requirements
The safety requirements of the equipment shall be in accordance with the provisions of GB 9706.1 and GB 9706.7.
5.9 Environmental test
The environmental test of the equipment shall be carried out in accordance with the methods and procedures specified in GB/T 14710 and Table 1.
6 Inspection rules
6.1 Inspection classification
Product inspection is divided into factory inspection and type inspection.
6.2 Factory inspection
The inspection items and determination rules for factory inspection shall be specified by the manufacturer, but shall at least include 4.1.
6.3 Type inspection
Type inspection should be carried out in one of the following cases.
a) new products are put into production;
b) When there are major changes in the design, process or materials that may cause changes in equipment performance.
The type test items are all items of this standard.
Get Quotation: Click YY/T 1090-2018 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1090-2018
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1090-2018: Ultrasonic physiotherapy equipment
YY/T 1090-2018
Ultrasonic physiotherapy equipment
ICS 11.040.60
C41
People's Republic of China Pharmaceutical Industry Standard
Replacing YY 1090-2009
Ultrasound therapy equipment
Published on.2018-12-20
2020-01-01 implementation
State Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY 1090-2009 "ultrasound physiotherapy equipment", compared with YY 1090-2009, except for editorial modification, the main technology
The changes are as follows.
---Modified the content of effective sound intensity (4.3, 4.2 of the.2009 edition);
--- Increased output power announcement (4.1,.2009 version 4.1.1);
--- Increased requirements for effective radiation area (4.2);
--- Increased the requirements for random files (4.7);
--- Removed the requirement to repeat with GB 9706.7-2008 (see 4.1.2, 4.1.3, 4.1.4, 4.1.5, 4.5 and 4.6 of the.2009 edition).
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Drug Administration.
This standard is under the jurisdiction of the National Medical Electrical Equipment Standardization Technical Committee Medical Ultrasound Equipment Subcommittee (SAC/TC10/SC2).
This standard was drafted. Hubei Medical Device Quality Supervision and Inspection Institute.
The main drafters of this standard. Jiang Shilin, Wang Zhiwei.
The previous versions of the standards replaced by this standard are.
---ZBC42012-1989;
---YY 91090-1999;
---YY/T 1090-2004;
---YY 1090-2009.
Ultrasound therapy equipment
1 Scope
This standard specifies the requirements, test methods and inspection rules for ultrasonic physiotherapy equipment.
This standard is applicable to continuous wave or quasi-continuous wave ultrasonic energy generated by a planar circular ultrasonic transducer in the frequency range of 0.5MHz~5MHz.
Amount of ultrasonic physiotherapy equipment (hereinafter referred to as equipment), this standard does not apply to effective sound stronger than 3W/cm2 or using focused ultrasound
device.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB 9706.1 Medical electrical equipment - Part 1. General requirements for safety
GB 9706.7 Medical electrical equipment - Part 2-5. Particular requirements for safety of physiotherapy equipment
GB/T 14710 Medical electrical equipment environmental requirements and test methods
YY/T 0750-2018 Sound field requirements and measurement methods in the frequency range of 0.5MHz~5MHz for ultrasonic physiotherapy equipment
YY/T 0865.1-2011 Ultrasonic hydrophones - Part 1. Measurement and characterization of medical ultrasound fields below 40 MHz
3 Terms and definitions
The terms and definitions defined in GB 9706.7 and YY/T 0750 apply to this document.
4 requirements
4.1 Accuracy of ultrasonic output power
The manufacturer shall publish the rated ultrasonic output power and the rated ultrasonic time maximum output power (if applicable), within its range, the power indication
The deviation of the value from the actual measured value shall not exceed ±20%.
4.2 Effective radiation area
The manufacturer shall publish the effective radiation area, and the deviation between the actual measured value and the published value shall not exceed the value published by the manufacturer.
4.3 Effective sound intensity
The manufacturer shall announce the maximum effective sound intensity at the nominal output power rating.
4.4 Acoustic operating frequency
The manufacturer shall announce the acoustic operating frequency, and the deviation between the actual measured value and the published value shall not exceed 10%.
4.5 Beam unevenness coefficient
RBN
The absolute maximum beam non-uniformity factor of all treatment heads or additional heads shall not exceed the manufacturer's published value and shall not exceed 8.0.
4.6 Appearance and structure
The appearance should be uniform in color, clean and tidy, free of scratches, cracks and other defects.
The text and logo on the panel should be legible and long-lasting.
The control and adjustment mechanism should be flexible and reliable, and the fastening parts are not loose.
4.7 Random files
The manufacturer shall publish the parameters to be published in 4.1~4.7 in the random file.
4.8 Safety requirements
The safety of the equipment shall comply with the requirements of GB 9706.1 and GB 9706.7.
4.9 Environmental test requirements
The manufacturer shall, in the random documents, the group of climatic environmental tests and mechanical environmental tests specified in GB/T 14710, test requirements and inspections.
The test items are implemented in accordance with Table 1.
Table 1 Environmental test requirements and inspection items
a Recovers at the recovery time specified by the manufacturer.
b Test according to the test conditions specified by the manufacturer.
c Test according to the test items specified by the manufacturer.
d Test voltage test according to the manufacturer's specifications.
5 Test methods
5.1 Accuracy of ultrasonic output power
The ultrasonic output power is carried out in accordance with the test methods specified in 7.2 and 7.3 of YY/T 0750-2018.
The rated ultrasonic output power and the rated ultrasonic time maximum output power shall be measured at 90%, 100% of the rated supply voltage, respectively.
Under the condition of 110%, it is not allowed to manually adjust the equipment during the measurement, take the most unfavorable value, and check whether the measurement result meets the requirements.
Measured at 1/3, 2/3 (or nearby, or gear) of the ultrasonic output power and the rated ultrasonic output maximum output power range, respectively.
The deviation between the indicated value of power and the actual measured value.
5.2 Effective radiation area
This is carried out in accordance with the test method specified in 7.4 of YY/T 0750-2018. The deviation is calculated according to equation (1).
5.3 Effective sound intensity
The effective sound intensity is calculated from the measured values of the output power and the effective radiation area.
5.4 Acoustic operating frequency
It is carried out in accordance with the test method specified in 7.3 of YY/T 0865.1-2011 and YY/T 0750-2018. The deviation is calculated according to equation (1).
5.5 Beam non-uniformity coefficient
This is carried out in accordance with the test method specified in 7.4 of YY/T 0750-2018.
5.6 Appearance and structure
Visual inspection should meet the requirements of 4.7.
5.7 Random files
Check random documents and meet the requirements of 4.8.
5.8 Safety requirements
The safety requirements of the equipment shall be in accordance with the provisions of GB 9706.1 and GB 9706.7.
5.9 Environmental test
The environmental test of the equipment shall be carried out in accordance with the methods and procedures specified in GB/T 14710 and Table 1.
6 Inspection rules
6.1 Inspection classification
Product inspection is divided into factory inspection and type inspection.
6.2 Factory inspection
The inspection items and determination rules for factory inspection shall be specified by the manufacturer, but shall at least include 4.1.
6.3 Type inspection
Type inspection should be carried out in one of the following cases.
a) new products are put into production;
b) When there are major changes in the design, process or materials that may cause changes in equipment performance.
The type test items are all items of this standard.
Share