1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1288-2015 English PDF (YY/T1288-2015)
YY/T 1288-2015 English PDF (YY/T1288-2015)
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1288-2015 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1288-2015
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1288-2015: Nylon blood filter nets for transfusion equipment for single use
YY/T 1288-2015
Nylon blood filter nets for transfusion equipments for single use
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Single use blood transfusion apparatus with nylon blood filter
Published on.2015-03-02
2016-01-01 Implementation
The State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. Publication of this document
The agency does not assume responsibility for identifying these patents.
This standard is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
This standard is mainly drafted by. Wuxi Bolcon Medical Machinery Plastic Co., Ltd.
Participated in the drafting of this standard. Shandong Medical Device Product Quality Inspection Center, Shanghai Zhenpu Medical Equipment Co., Ltd., Jintan Kang
Da Medical Equipment Manufacturing Co., Ltd.
The main drafters of this standard. Zhang Xianshun, Guo Maochun, Jia Yufei, Zhang Songhe, Shi Lijun, Liu Lili, Wang Tongchao, Zhang Bo, and Guo Xianhu.
Single use blood transfusion apparatus with nylon blood filter
1 Scope
This standard specifies the requirements for a disposable blood transfusion apparatus used for nylon blood filters (hereinafter referred to as filters). Filters can be installed on
It is used to filter blood clots, impurities and foreign matter in blood or blood products.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB 8369 One-time use of blood transfusion device
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB/T 19466.3-2004 Plastics - Differential scanning calorimetry (DSC) - Part 3. Determination of melting and crystallization temperature and enthalpy
Pharmacopoeia of the People's Republic of China (Part 2).2010 Edition
3 Classification and marking
3.1 The structure of the filter
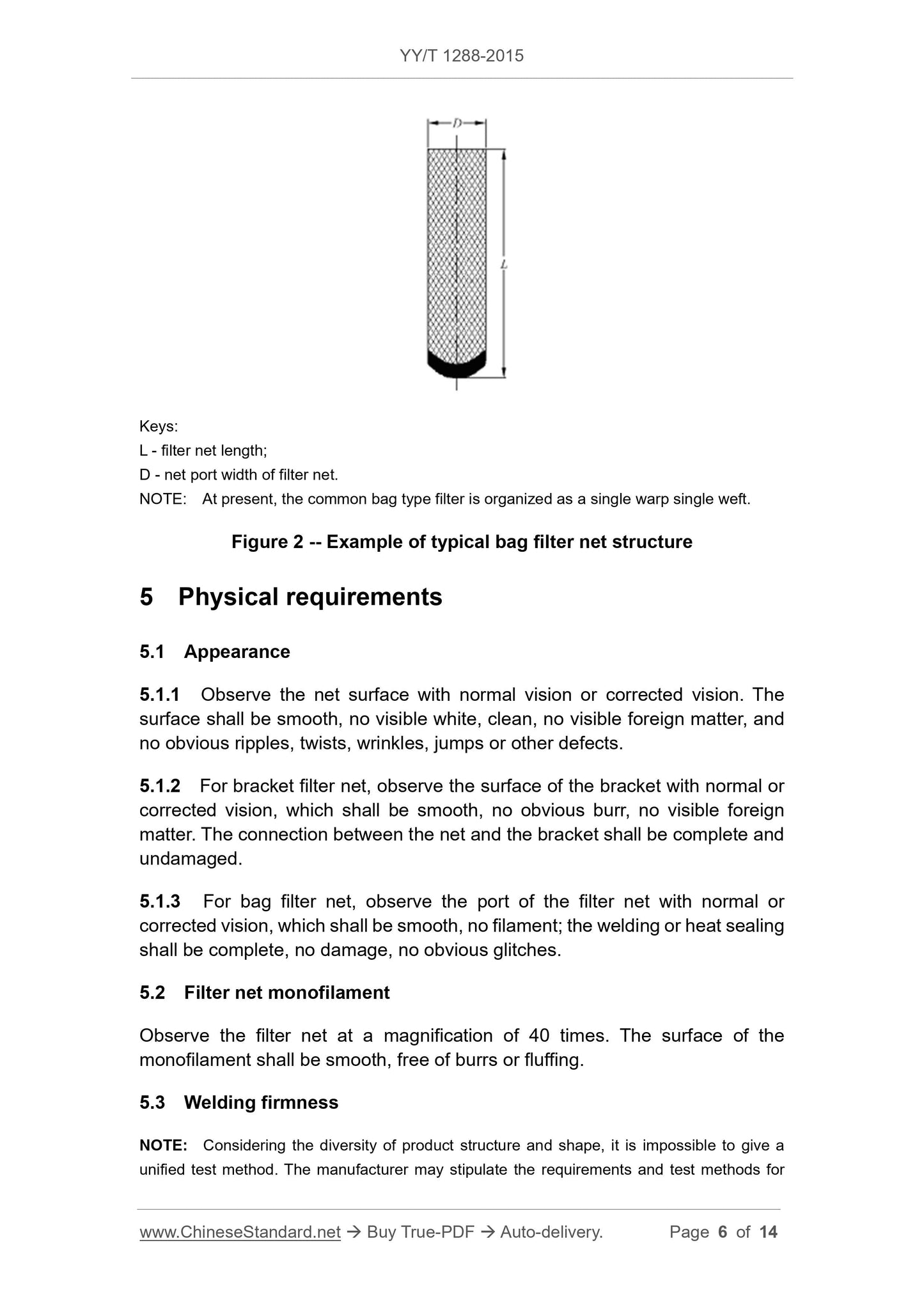
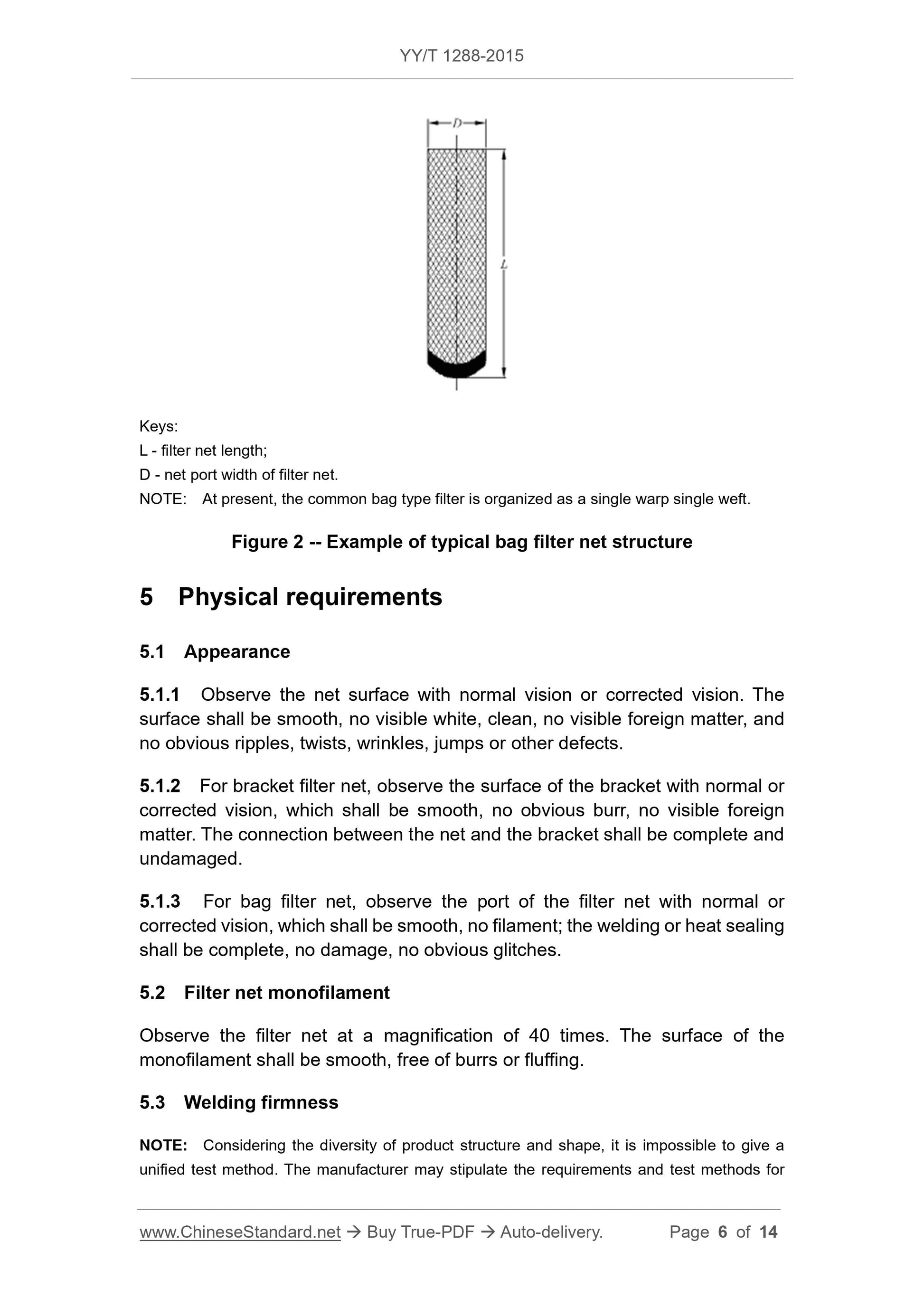
An example of a typical stent-type filter structure is shown in Fig. 1, and a typical bag-type filter (non-fixed stent) structure is shown in Fig. 2.
3.2 Marking
Examples of filter tags are as follows.
The bracket filter with an outer diameter of φ16 mm and a bracket length of 45 mm is marked with.
Φ16×45
The label of the bag filter with a mesh width of 16 mm and a filter length of 45 mm is.
16×45
4 Materials
4.1 The mesh of the filter should be woven from nylon 66 and nylon 1010 monofilaments.
4.2 The filter material (including the material of the bracket) shall also meet the requirements of Chapters 5, 6 and 7.
Explanation.
1 --- bracket;
2---filter;
L---stent length;
D --- filter network port diameter.
Note. At present, common stent-type filters are organized in single warp and single weft.
Figure 1 Typical bracket filter structure example
Explanation.
L --- filter length;
D---filter network port width.
Note. At present, common bag filter forms are single-double longitude.
Figure 2 Typical bag filter structure example
5 Physical requirements
5.1 Appearance
5.1.1 Observe the mesh surface with normal vision or corrected vision. The surface of the mesh should be flat, no visible white, clean, no visible foreign matter, and no obvious waves.
Patterns, twists, wrinkles, jumps and other defects.
5.1.2 For the stent-type filter, observe the surface of the stent with normal vision or corrected vision should be smooth, no obvious burr, no visible difference
The connection between the object, net and bracket shall be complete and undamaged.
5.1.3 For bag filters, the mesh of the filter should be observed with normal vision or corrected vision. There should be no dehairing, welding or heat.
The joint should be complete, without damage, no obvious glitches.
5.2 Filament Filament
Observe the filter at a magnification of 40 times. The surface of the monofilament should be smooth, free of burrs or fluffing.
5.3 Welding firmness
Note. Due to the diversity of product structure and shape, it is impossible to give a unified test method. Manufacturers can stipulate requirements for welding firmness and
experiment method.
5.4 Particle Pollution
The filter should be made under conditions of minimal particulate contamination. When tested in accordance with Appendix A, the pollution index should not exceed 90.
5.5 Filtration Performance
When tested according to Appendix B, the rejection of the 240 μm particles by the filter should not be less than 95%, and the pass rate of the 160 μm particles should not be small.
At 95%.
Note. If tested, the test filter is organized in the form of single warp and weft, with a wire diameter of 100 μm ± 10 μm and an aperture diameter of.200 μm ± 20 μm.
Do not filter performance test.
5.6 melting peak temperature
When tested according to the method specified in GB/T 19466.3, the monofilament melting peak temperature (melting point) of a filter made of nylon 66 monofilaments
Should be in the range of 246°C ~ 257°C (475°F ~ 495°F); the monofilament melting peak temperature of a filter made of nylon 1010 monofilament
(melting point) should be in the range of 198°C to 210°C (388°F to 410°F).
Note. Other validated equivalent test methods can also be used for measurement.
6 Chemical requirements
6.1 Preparation of Chemical Performance Test Liquid
Take several samples, add the glass container, add water according to the ratio of the total internal surface area (cm2) and water (mL) of the sample is 2.1.
After the lid was placed, the sample was placed at 37°C±1°C for 24 hours, and the sample was separated from the liquid and cooled to room temperature as a test liquid. Take the same volume of water in the glass
In the device, a blank control solution was prepared in the same manner.
6.2 Reducing substances
When testing according to 5.2.2 of GB/T 14233.1-2008, the test solution and blank solution consume potassium permanganate solution.
The difference in volume of c(KMnO4)=0.002 mol/L should not exceed 2.0 mL.
6.3 Metal ions
When tested according to 5.9.1 Atomic Absorption Spectrophotometry (AAS) or equivalent method in GB/T 14233.1-2008, the test solution
The total content of germanium, chromium, copper, lead and tin should not exceed 1 μg/mL, and the content of cadmium should not exceed 0.1 μg/mL.
According to the colorimetric test of 5.6.1 in GB/T 14233.1-2008, the color of the test solution should not exceed the mass concentration
ρ(Pb2)=1 μg/mL standard control.
6.4 pH Titrations
When testing according to the method of 5.4.2 of GB/T 14233.1-2008, any standard solution required to make the indicator color gray
Not more than 1 mL.
6.5 Evaporation residue
When tested according to the method of 5.5 in GB/T 14233.1-2008, the total amount of evaporation residues should not exceed 2 mg.
6.6 UV absorbance
According to the test method of 5.7 in GB/T 14233.1-2008, the absorbance of the test solution for the wavelength range of 250nm~320nm should be
Not more than 0.1.
6.7 Fluorescent substances
Ten randomly selected filters of the same batch were placed under a 365 nm UV lamp and should not show strong blue fluorescence.
7 Biological requirements
7.1 Biocompatibility
The biological evaluation of the filter should be carried out in accordance with GB/T 16886.1. The evaluation results should show that there is no biological hazard.
7.2 Microbial limits
According to the "People's Republic of China Pharmacopoeia (Part II)".2010 edition appendix XIJ regulations, weigh 10g sample, add pH7.0 sterile sodium chloride -
A 100-mL peptone buffer solution was used to prepare the extract. The membrane filtration method was used for the test. The total number of bacteria should not exceed.200 CFU/g.
8 type inspection
8.1 Type test is a full-performance test.
8.2 In the type inspection, if there is no special provision, the physical requirements shall be randomly sampled by 5, and other tests shall be conducted according to the standard. If all check items
If all the items are qualified, it is judged as qualified, otherwise it is judged as unqualified.
9 signs
9.1 Initial packaging marks
The following symbols should be on the initial packaging of the product.
a) Manufacturer's name and/or trademark, address, contact information;
b) product name and specification model;
c) the material and organization of the filter;
d) quantity;
e) product effective date;
f) batch number;
g) This standard number.
9.2 Packaging Marks
The following symbols should be on the outer packaging.
a) Manufacturer's name and/or trademark, address, contact information;
b) product name and specification model;
c) the material and organization of the filter;
d) Net weight, gross weight, quantity;
e) volume (length x height x width);
f) the effective date of the product;
g) lot number;
h) this standard number;
i) Words and symbols such as “Handle with care,” “Heat to heat,” “Heat to wet,” etc.
10 Packaging
When a specified number of filters are loaded into a package (at least double layer), the packaging material must not have harmful effects on the contents and ensure that
The contents are not contaminated.
Appendix A
(Normative Appendix)
Filter particle contamination test method
A.1 Test Equipment
A.1.1 Particle Counter. Including resistance or photoresist, with stirring system, a sampling of not less than 1mL.
A.1.2 Rinsing solution. water or sodium chloride solution with a mass concentration of 9 g/L 1) filtered through a microporous membrane with a pore size of 0.2 μm, 5 μm or more
The number of particles is not more than 10 particles/mL.
A.2 Preparation of test solution
Use a non-contaminating method to immerse 10 filters in a conical beaker with 500 mL of rinse solution (A.1.2) and apply suitable materials.
(such as aluminum foil) Cover the tapered beaker cup, then place the cone beaker on the oscillator (horizontal rotation, oscillation frequency 300r/min ±
10r/min) oscillate for 20s, carefully remove the sealing material, then use a non-polluting method, remove the filter, and stand for 5min after washing
Deliquoring.
Another 500 mL rinse (A.1.2) was used as a blank control.
A.3 Test methods
According to the method stipulated in GB 8369, the total number of particles in the 10 blood filters in the eluent and the number of particles in the blank control solution were checked and counted.
Calculate the pollution index.
1) Use this solution when using a resistive particle counter.
Appendix B
(Normative Appendix)
Filter filtration performance test method
B.1 General
The test should be conducted in a clean environment and, if possible, under laminar flow.
B.2 Test solution
Prepare a 240 μm particle test solution with a concentration of approximately.200 particles/100 mL and a particle size of 160 μm using particles 2) that meet the requirements of Table B.1.
Particle test solution.
Table B.1 Particles Technical Requirements
Nominal size
Μm
Average particle size
Μm
Standard deviation of particle size
Μm
240 239±4.8 ≤9.0
160 158±2.2 ≤3.5
B.3 rinse solution
Filtration through a 0.2 μm pore size microfiltration membrane, water with a particle size of 5 μm or more and no more than 10 particles/mL.
B.4 Retention of 240 μm particles
B.4.1 Test procedure
B.4.1.1 Take 100ml of 240μm test solution (B.2) from the measuring cylinder, and let the test solution (B.2) flow through the non-contaminating method in the transfusion direction.
The mesh was screened and the effluent was passed through a single grid filter having a pore size of 0.8 μm and a diameter of 47 mm.
B.4.1.2 Take a suitable amount (not less than 100 mL) of the rinsing solution (B.3) with the same cylinder, and allow the rinsing solution to flow through the same filter in the transfusion direction.
The web was passed through the same grid filter with a diameter of 0.8 μm and a diameter of 47 mm.
B.4.1.3 Place the grid filter with the particles on a suitable microscope slide or tray, at a magnification of 40 times
The number of particles in the 50% of the grid area is counted. Obvious non-test particles do not count. The test was performed twice.
2) ThermoScientific's 4324A and 4316A particles are examples of suitable commercial products. This information is given to facilitate the use of this standard.
The user does not mean that this product is recognized.
B.4.2 Result representation
The rejection of the filter for 240 μm particles was calculated according to equation (B.1).
η = (1-N1N0) × 100% (B.1)
In the formula.
η ---retention rate,%;
N0 --- the number of particles measured in the test solution, in units;
N1---The number of particles on the grid filter, in units.
B.5 Passing rate of 160μm particles
B.5.1 Test procedure
B.5.1.1 Take 100ml of 160μm test solution (B.2) from the measuring cylinder, and let the test solution (B.2) flow through the non-contaminating method in the transfusion direction.
Filter, and then use the same measuring cylinder to take the appropriate amount (not less than 100mL) of the rinsing solution (B.3), so that the rinsing fluid flows in the same direction according to the transfusion direction.
Strainer.
B.5.1.2 clamp the filter network port with tweezers and place it in a beaker with a suitable amount of rinse solution (B.3). The filter network port is basically liquid
The surface is flat or slightly above the liquid level, and then the filter mesh is reciprocated horizontally in the beaker at least 5 times (maintaining the filter net mouth is basically the same as the liquid surface or
Slightly above the liquid level) to elute particles adhering to the outside of the filter, remove the filter and discard the eluent in the beaker. Repeat this operation once.
Note. Particles adhering to the outside of the filte...
Get Quotation: Click YY/T 1288-2015 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1288-2015
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1288-2015: Nylon blood filter nets for transfusion equipment for single use
YY/T 1288-2015
Nylon blood filter nets for transfusion equipments for single use
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Single use blood transfusion apparatus with nylon blood filter
Published on.2015-03-02
2016-01-01 Implementation
The State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. Publication of this document
The agency does not assume responsibility for identifying these patents.
This standard is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
This standard is mainly drafted by. Wuxi Bolcon Medical Machinery Plastic Co., Ltd.
Participated in the drafting of this standard. Shandong Medical Device Product Quality Inspection Center, Shanghai Zhenpu Medical Equipment Co., Ltd., Jintan Kang
Da Medical Equipment Manufacturing Co., Ltd.
The main drafters of this standard. Zhang Xianshun, Guo Maochun, Jia Yufei, Zhang Songhe, Shi Lijun, Liu Lili, Wang Tongchao, Zhang Bo, and Guo Xianhu.
Single use blood transfusion apparatus with nylon blood filter
1 Scope
This standard specifies the requirements for a disposable blood transfusion apparatus used for nylon blood filters (hereinafter referred to as filters). Filters can be installed on
It is used to filter blood clots, impurities and foreign matter in blood or blood products.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB 8369 One-time use of blood transfusion device
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB/T 19466.3-2004 Plastics - Differential scanning calorimetry (DSC) - Part 3. Determination of melting and crystallization temperature and enthalpy
Pharmacopoeia of the People's Republic of China (Part 2).2010 Edition
3 Classification and marking
3.1 The structure of the filter
An example of a typical stent-type filter structure is shown in Fig. 1, and a typical bag-type filter (non-fixed stent) structure is shown in Fig. 2.
3.2 Marking
Examples of filter tags are as follows.
The bracket filter with an outer diameter of φ16 mm and a bracket length of 45 mm is marked with.
Φ16×45
The label of the bag filter with a mesh width of 16 mm and a filter length of 45 mm is.
16×45
4 Materials
4.1 The mesh of the filter should be woven from nylon 66 and nylon 1010 monofilaments.
4.2 The filter material (including the material of the bracket) shall also meet the requirements of Chapters 5, 6 and 7.
Explanation.
1 --- bracket;
2---filter;
L---stent length;
D --- filter network port diameter.
Note. At present, common stent-type filters are organized in single warp and single weft.
Figure 1 Typical bracket filter structure example
Explanation.
L --- filter length;
D---filter network port width.
Note. At present, common bag filter forms are single-double longitude.
Figure 2 Typical bag filter structure example
5 Physical requirements
5.1 Appearance
5.1.1 Observe the mesh surface with normal vision or corrected vision. The surface of the mesh should be flat, no visible white, clean, no visible foreign matter, and no obvious waves.
Patterns, twists, wrinkles, jumps and other defects.
5.1.2 For the stent-type filter, observe the surface of the stent with normal vision or corrected vision should be smooth, no obvious burr, no visible difference
The connection between the object, net and bracket shall be complete and undamaged.
5.1.3 For bag filters, the mesh of the filter should be observed with normal vision or corrected vision. There should be no dehairing, welding or heat.
The joint should be complete, without damage, no obvious glitches.
5.2 Filament Filament
Observe the filter at a magnification of 40 times. The surface of the monofilament should be smooth, free of burrs or fluffing.
5.3 Welding firmness
Note. Due to the diversity of product structure and shape, it is impossible to give a unified test method. Manufacturers can stipulate requirements for welding firmness and
experiment method.
5.4 Particle Pollution
The filter should be made under conditions of minimal particulate contamination. When tested in accordance with Appendix A, the pollution index should not exceed 90.
5.5 Filtration Performance
When tested according to Appendix B, the rejection of the 240 μm particles by the filter should not be less than 95%, and the pass rate of the 160 μm particles should not be small.
At 95%.
Note. If tested, the test filter is organized in the form of single warp and weft, with a wire diameter of 100 μm ± 10 μm and an aperture diameter of.200 μm ± 20 μm.
Do not filter performance test.
5.6 melting peak temperature
When tested according to the method specified in GB/T 19466.3, the monofilament melting peak temperature (melting point) of a filter made of nylon 66 monofilaments
Should be in the range of 246°C ~ 257°C (475°F ~ 495°F); the monofilament melting peak temperature of a filter made of nylon 1010 monofilament
(melting point) should be in the range of 198°C to 210°C (388°F to 410°F).
Note. Other validated equivalent test methods can also be used for measurement.
6 Chemical requirements
6.1 Preparation of Chemical Performance Test Liquid
Take several samples, add the glass container, add water according to the ratio of the total internal surface area (cm2) and water (mL) of the sample is 2.1.
After the lid was placed, the sample was placed at 37°C±1°C for 24 hours, and the sample was separated from the liquid and cooled to room temperature as a test liquid. Take the same volume of water in the glass
In the device, a blank control solution was prepared in the same manner.
6.2 Reducing substances
When testing according to 5.2.2 of GB/T 14233.1-2008, the test solution and blank solution consume potassium permanganate solution.
The difference in volume of c(KMnO4)=0.002 mol/L should not exceed 2.0 mL.
6.3 Metal ions
When tested according to 5.9.1 Atomic Absorption Spectrophotometry (AAS) or equivalent method in GB/T 14233.1-2008, the test solution
The total content of germanium, chromium, copper, lead and tin should not exceed 1 μg/mL, and the content of cadmium should not exceed 0.1 μg/mL.
According to the colorimetric test of 5.6.1 in GB/T 14233.1-2008, the color of the test solution should not exceed the mass concentration
ρ(Pb2)=1 μg/mL standard control.
6.4 pH Titrations
When testing according to the method of 5.4.2 of GB/T 14233.1-2008, any standard solution required to make the indicator color gray
Not more than 1 mL.
6.5 Evaporation residue
When tested according to the method of 5.5 in GB/T 14233.1-2008, the total amount of evaporation residues should not exceed 2 mg.
6.6 UV absorbance
According to the test method of 5.7 in GB/T 14233.1-2008, the absorbance of the test solution for the wavelength range of 250nm~320nm should be
Not more than 0.1.
6.7 Fluorescent substances
Ten randomly selected filters of the same batch were placed under a 365 nm UV lamp and should not show strong blue fluorescence.
7 Biological requirements
7.1 Biocompatibility
The biological evaluation of the filter should be carried out in accordance with GB/T 16886.1. The evaluation results should show that there is no biological hazard.
7.2 Microbial limits
According to the "People's Republic of China Pharmacopoeia (Part II)".2010 edition appendix XIJ regulations, weigh 10g sample, add pH7.0 sterile sodium chloride -
A 100-mL peptone buffer solution was used to prepare the extract. The membrane filtration method was used for the test. The total number of bacteria should not exceed.200 CFU/g.
8 type inspection
8.1 Type test is a full-performance test.
8.2 In the type inspection, if there is no special provision, the physical requirements shall be randomly sampled by 5, and other tests shall be conducted according to the standard. If all check items
If all the items are qualified, it is judged as qualified, otherwise it is judged as unqualified.
9 signs
9.1 Initial packaging marks
The following symbols should be on the initial packaging of the product.
a) Manufacturer's name and/or trademark, address, contact information;
b) product name and specification model;
c) the material and organization of the filter;
d) quantity;
e) product effective date;
f) batch number;
g) This standard number.
9.2 Packaging Marks
The following symbols should be on the outer packaging.
a) Manufacturer's name and/or trademark, address, contact information;
b) product name and specification model;
c) the material and organization of the filter;
d) Net weight, gross weight, quantity;
e) volume (length x height x width);
f) the effective date of the product;
g) lot number;
h) this standard number;
i) Words and symbols such as “Handle with care,” “Heat to heat,” “Heat to wet,” etc.
10 Packaging
When a specified number of filters are loaded into a package (at least double layer), the packaging material must not have harmful effects on the contents and ensure that
The contents are not contaminated.
Appendix A
(Normative Appendix)
Filter particle contamination test method
A.1 Test Equipment
A.1.1 Particle Counter. Including resistance or photoresist, with stirring system, a sampling of not less than 1mL.
A.1.2 Rinsing solution. water or sodium chloride solution with a mass concentration of 9 g/L 1) filtered through a microporous membrane with a pore size of 0.2 μm, 5 μm or more
The number of particles is not more than 10 particles/mL.
A.2 Preparation of test solution
Use a non-contaminating method to immerse 10 filters in a conical beaker with 500 mL of rinse solution (A.1.2) and apply suitable materials.
(such as aluminum foil) Cover the tapered beaker cup, then place the cone beaker on the oscillator (horizontal rotation, oscillation frequency 300r/min ±
10r/min) oscillate for 20s, carefully remove the sealing material, then use a non-polluting method, remove the filter, and stand for 5min after washing
Deliquoring.
Another 500 mL rinse (A.1.2) was used as a blank control.
A.3 Test methods
According to the method stipulated in GB 8369, the total number of particles in the 10 blood filters in the eluent and the number of particles in the blank control solution were checked and counted.
Calculate the pollution index.
1) Use this solution when using a resistive particle counter.
Appendix B
(Normative Appendix)
Filter filtration performance test method
B.1 General
The test should be conducted in a clean environment and, if possible, under laminar flow.
B.2 Test solution
Prepare a 240 μm particle test solution with a concentration of approximately.200 particles/100 mL and a particle size of 160 μm using particles 2) that meet the requirements of Table B.1.
Particle test solution.
Table B.1 Particles Technical Requirements
Nominal size
Μm
Average particle size
Μm
Standard deviation of particle size
Μm
240 239±4.8 ≤9.0
160 158±2.2 ≤3.5
B.3 rinse solution
Filtration through a 0.2 μm pore size microfiltration membrane, water with a particle size of 5 μm or more and no more than 10 particles/mL.
B.4 Retention of 240 μm particles
B.4.1 Test procedure
B.4.1.1 Take 100ml of 240μm test solution (B.2) from the measuring cylinder, and let the test solution (B.2) flow through the non-contaminating method in the transfusion direction.
The mesh was screened and the effluent was passed through a single grid filter having a pore size of 0.8 μm and a diameter of 47 mm.
B.4.1.2 Take a suitable amount (not less than 100 mL) of the rinsing solution (B.3) with the same cylinder, and allow the rinsing solution to flow through the same filter in the transfusion direction.
The web was passed through the same grid filter with a diameter of 0.8 μm and a diameter of 47 mm.
B.4.1.3 Place the grid filter with the particles on a suitable microscope slide or tray, at a magnification of 40 times
The number of particles in the 50% of the grid area is counted. Obvious non-test particles do not count. The test was performed twice.
2) ThermoScientific's 4324A and 4316A particles are examples of suitable commercial products. This information is given to facilitate the use of this standard.
The user does not mean that this product is recognized.
B.4.2 Result representation
The rejection of the filter for 240 μm particles was calculated according to equation (B.1).
η = (1-N1N0) × 100% (B.1)
In the formula.
η ---retention rate,%;
N0 --- the number of particles measured in the test solution, in units;
N1---The number of particles on the grid filter, in units.
B.5 Passing rate of 160μm particles
B.5.1 Test procedure
B.5.1.1 Take 100ml of 160μm test solution (B.2) from the measuring cylinder, and let the test solution (B.2) flow through the non-contaminating method in the transfusion direction.
Filter, and then use the same measuring cylinder to take the appropriate amount (not less than 100mL) of the rinsing solution (B.3), so that the rinsing fluid flows in the same direction according to the transfusion direction.
Strainer.
B.5.1.2 clamp the filter network port with tweezers and place it in a beaker with a suitable amount of rinse solution (B.3). The filter network port is basically liquid
The surface is flat or slightly above the liquid level, and then the filter mesh is reciprocated horizontally in the beaker at least 5 times (maintaining the filter net mouth is basically the same as the liquid surface or
Slightly above the liquid level) to elute particles adhering to the outside of the filter, remove the filter and discard the eluent in the beaker. Repeat this operation once.
Note. Particles adhering to the outside of the filte...
Share