1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1291-2016 English PDF (YY/T1291-2016)
YY/T 1291-2016 English PDF (YY/T1291-2016)
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1291-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1291-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1291-2016: Single use subcutaneous infusion sets for use with insulin pump
YY/T 1291-2016
Single use subcutaneous infusion sets for use with insulin pump
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Disposable insulin pump subcutaneous infusion device
Published on.2016-03-23
2017-01-01 Implementation
The State Food and Drug Administration issued
Directory
Preface III
Introduction IV
1 Range 1
2 Normative references 1
3 Terms and Definitions 1
4 Structure and composition 2
5 Material 3
6 Physical Requirements 3
6.1 Line 3
6.1.1 Appearance 3
6.1.2 Transparency 3
6.1.3 Length 3
6.2 Hypodermic needle (if any) 3
6.2.1 Needle 3
6.2.2 Needle length 3
6.2.3 Needle bend direction and angle 3
6.2.4 Tip 4
6.2.5 needle handle connection firmness 4
6.3 Subcutaneous trocar (if any) 4
6.3.1 Conduit length 4
6.3.2 Catheter and catheter base connection firmness 4
6.3.3 Puncture needle 4
6.3.4 Fitting length of puncture needle and catheter 4
6.4 Separators (if any) 4
6.4.1 Self-sealing 4
6.4.2 Easy Connectivity 4
6.4.3 Locking device 4
6.5 Adhesive tape (if any) 4
6.5.1 release paper/separator 4
6.5.2 Peeling Strength of Integrated Adhesive Tape 4
6.5.3 Separating adhesive tape peel strength 5
6.5.4 Water vapor permeability 5
6.6 Interface 5
6.7 Pipe Connection Strength 5
6.8 Corrosion resistance 5
6.9 Particle Pollution 5
6.10 Leak 5
6.11 Smoothness 5
6.12 Stability 5
6.12.1 resistance to external interference 5
6.12.2 Flex resistance 5
6.13 pill volume 6
6.14 protective cover 6
7 Chemical properties 6
7.1 Preparation of test solution 6
7.2 Reducing substances 6
7.3 Metal Ions 6
7.4 pH Titrations 6
7.5 evaporation residue 6
7.6 UV absorbance 6
8 Biological properties 6
8.1 Biocompatibility 6
8.2 Sterile 7
8.3 Bacterial endotoxin 7
9 Type Inspection 7
10 Signs 7
10.1 Single Pack 7
10.2 Attached Document 7
10.3 Packing 8
10.4 Transport Package 8
11 Packing 9
Appendix A (Normative) Physical Test 10
Appendix B (Informative) Design Guidelines for Subcutaneous Infusion Sets 13
Reference 14
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This standard is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
This standard is mainly drafted by. Shandong Provincial Medical Device Product Quality Inspection Center.
Participated in the drafting of this standard. Zhengzhou Ruiyu Technology Co., Ltd., Medtronic (Shanghai) Management Co., Ltd.
The main drafters of this standard. Liu Lili, Yao Xiujun, Wang Yongxin, Wang Jinhong, and Jia Yufei.
introduction
The disposable infusion pump for disposable insulin pump is generally composed of an interface, a pipeline, and a puncturing component, and is connected with a drug reservoir contained in an insulin pump.
Then, the micro-computer controls the injection of exogenous insulin on time, quantitatively and accurately to the user's skin, keeping the blood sugar level for 24 hours stable.
Control the purpose of diabetes.
This standard will be divided into subcutaneous needle and subcutaneous trocar.
This standard does not classify subcutaneous infusion sets for disposable insulin pumps.
This standard provides design guidelines in the form of informative annexes (Appendix B).
This standard stipulates the requirements for single-use subcutaneous infusion sets for insulin pumps and provides a unified evaluation of disposable insulin pumps.
Use the performance indicators and test methods for the stability of subcutaneous infusion sets.
Disposable insulin pump subcutaneous infusion device
1 Scope
This standard specifies the requirements for subcutaneous infusion sets for insulin pumps (referred to as "subcutaneous infusion sets") consisting of interfaces, tubing and piercing components.
This product is a single use sterile product.
This standard does not include the requirements for insulin-filled devices (eg, drug reservoirs, pre-filled cartridges) in insulin pumps.
This standard does not address the accuracy requirements for flow control when a subcutaneous infusion set is fitted with an insulin pump.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 1962.2 Syringes, injection needles and other medical devices 6% (Luer) conical joints Part 2. Locking joints
(GB/T 1962.2-2001, ISO 594-2.1998, IDT)
GB 8368-2005 Single-use infusion set for gravity infusion (ISO 8536-4.2004, MOD)
GB 9706.27 Medical electrical equipment - Part 22-24. Particular requirements for the safety of infusion pumps and infusion controllers (GB 9706.27-
2005, IEC 60601-2-24.1998, IDT)
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 14233.2-2005 Methods for the examination of medical infusions, blood transfusions and syringes - Part 2. Biological methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Evaluation and testing in risk management process (GB/T 16886.1-
2011, ISO 10993-1.2009, IDT)
GB 18457 Stainless steel needles for medical devices (GB 18457-2001, ISO 9626.1991, IDT)
YY/T 0148-2006 Medical Tape General Requirements
YY 0285.1-2004 Disposable sterile endovascular catheters Part 1. General requirements (ISO 10555-1.1995, IDT)
YY 0285.5-2004 Disposable sterile intravascular catheters Part 5. Trocar peripheral catheters (ISO 10555-5.1996,
IDT)
YY/T 0466.1 Medical Devices Symbols for Medical Devices Signs, Labels, and Information Provided (YY/T 0466.1-2009,
ISO 15223-1.2007, IDT)
YY/T 0615.1 Requirements for Labeling “Aseptic” Medical Devices Part 1. Requirements for Final Sterilization Medical Devices
ISO 11607-1.2006 Packaging for terminally sterilized medical devices Part 1. Requirements for materials, sterile barrier systems, and packaging systems 1)
1) The Chinese standard GB/T 19633.1 equivalent to ISO 11607-1.2006 is currently in the approval stage.
3 Terms and Definitions
The following terms and definitions apply to this document.
3.1
Piercing component introducerneedleunit
Subcutaneous infusion device for subcutaneous puncture components, usually subcutaneous needle (or subcutaneous trocar), needle handle (or catheter base), fixed paste
Tape composition.
3.2
Hypodermic needlenee
Rigid tube with cutting edge on one end for subcutaneous administration, typically with oblique insertion and in-line insertion
3.3
Subcutaneous trocar hypodermicneedleandcatheterassembly
An assembly consisting of a metal piercing needle and a catheter over it. After the subcutaneous trocar is punctured into the skin, the metal puncture needle is pulled out.
Placed subcutaneously for subcutaneous administration.
3.4
Separator segregator
An assembly on the tubing near the piercing assembly for disconnecting and connecting the piercing assembly from the insulin pump.
Note. The purpose of the separator is to facilitate the use of people who need to temporarily remove the insulin pump.
3.5
Interface connector
The connection between the subcutaneous infusion set and the reservoir of the insulin pump.
3.6
Fixed surface adhesivesurface
Attach the adhesive tape to the adhesive side.
3.7
Fixed segment fixedsegment
A subcutaneous infusion set with a separator is from the piercing assembly to the section between the outlets of the separator.
3.8
Movable segment removesegment
The subcutaneous infusion set with the separator is from the separator head to the interface between the connectors.
4 Structure and composition
The structure and composition of a typical subcutaneous infusion set are shown in Figure 1.
Explanation.
1---Paste adhesive tape for puncture assembly (integrated) a;
2 --- needle handle;
3 --- subcutaneous needle b;
4---pipeline;
5---Separator socket c;
6---plug c of the splitter;
7---Separator adhesive tape (integral) d;
8---pipeline;
9--- interface.
a can also be a separate adhesive tape provided separately;
b. The hypodermic needle may also be the structure of the trocar shown in Fig. 2;
c is an optional component;
d can also be a separate adhesive tape provided separately.
Fig.1 Schematic diagram of subcutaneous infusion set for insulin pump
Note. Figure 1 is for illustration purposes only and is not the only type specified in this standard.
Explanation.
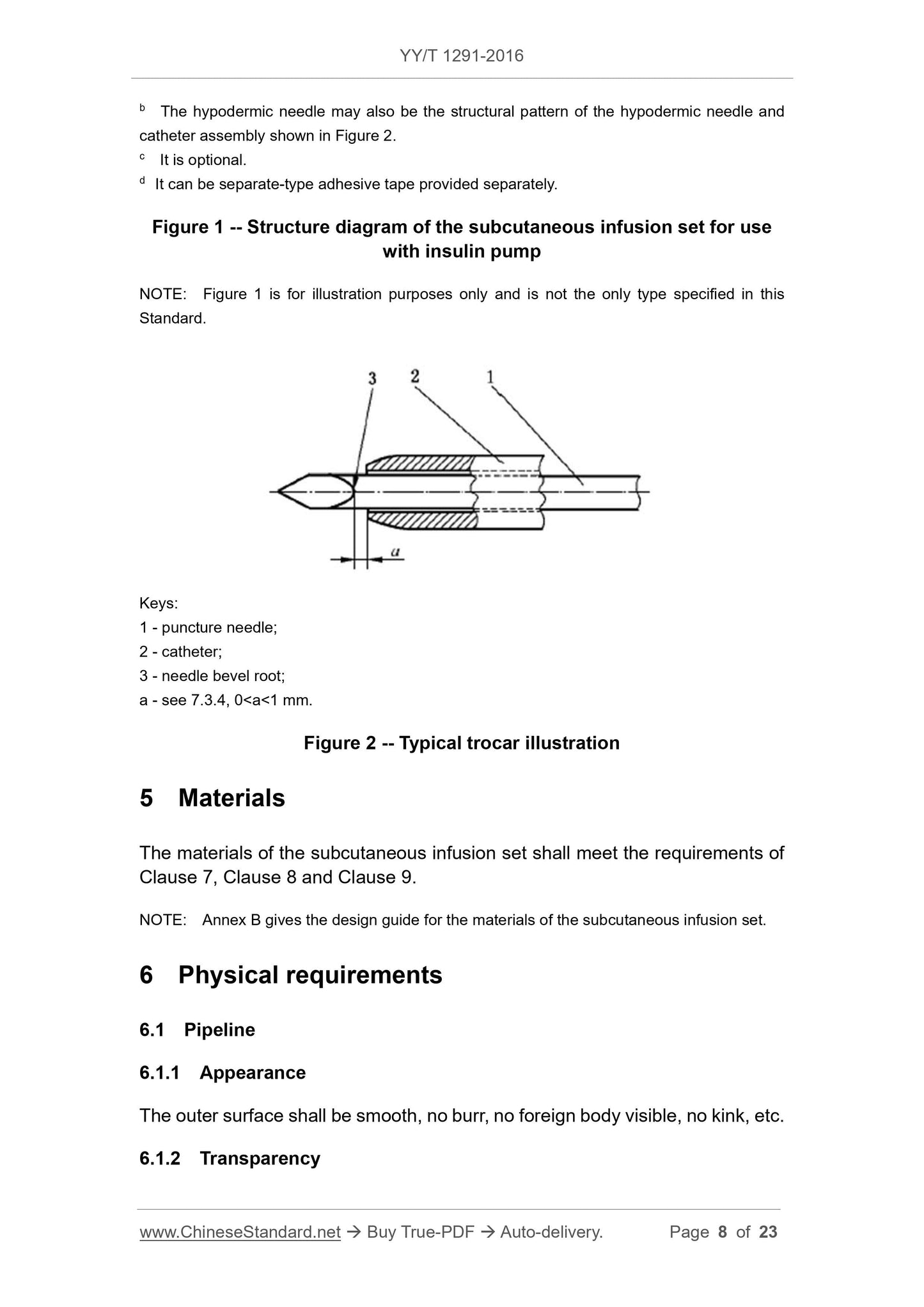
1 --- puncture needle;
2 --- conduit;
3 --- the root of the needle tip slope;
a--- see 7.3.4,0 \u003ca\u003c1mm。
Figure 2 Typical trocar illustration
5 Materials
The material of the subcutaneous infusion set should meet the requirements specified in Chapter 7, Chapter 8, and Chapter 9.
Note. Appendix B provides design guidelines for subcutaneous infusion set materials.
6 Physical requirements
6.1 Pipeline
6.1.1 Appearance
The outer surface should be smooth, free from burrs, visible to the naked eye, and free from kinks.
6.1.2 Transparency
It should be transparent or sufficiently transparent. When tested according to A.1, it should be able to detect the gas-liquid interface.
6.1.3 Length
The length of the pipeline should not be less than 90% of the nominal value.
6.2 Hypodermic needle (if any)
6.2.1 Needle
The needle section and the wall thickness should be uniform, and the liquid passage of the needle should be clear.
6.2.2 Needle length
The effective length of the needle tube should meet the requirements of the nominal value, and the tolerance is ±1mm.
Note. The effective length of the needle is the length of the first bend from the needle tip to the needle. For in-line hypodermic needles, the first bend is the needle and wear
The thorn component is pasted at the junction of the tape, ie, the bending angle is close to 90°; for the oblique insertion type hypodermic needle, the first bend is exposed at the outside bending point.
Discounts.
6.2.3 Needle bend direction and angle
When the needle bending angle is visually observed parallel to the fixing surface of the needle shank and the direction of the tube (in the direction shown in Fig. 1), the needle tube and the needle
The angle on the pipe side of the shank should be equal to or greater than 90°.
6.2.4 Tip
With normal vision or corrected vision, the needle tip should be sharp, free from kinks, nicks, and hooks when examined under magnification 2.5 times.
6.2.5 Fastness of needle connection
The static tensile force of 10 N applied for 10 seconds between the hypodermic needle and the handle is not to be broken or loosened.
6.3 Subcutaneous trocar (if any)
6.3.1 Catheter length
The effective length of the catheter (the length from the tip of the catheter to the end of the catheter) should be expressed in millimeters. The effective length of the catheter should meet the nominal value
The tolerance is ±1mm.
6.3.2 The connection between catheter and catheter base
The connection between the catheter and the base of the catheter should be able to withstand a static axial tension of 3 N for 15 s and there should be no loosening or separation of the connection.
6.3.3 Needle
With normal vision or corrected vision, the needle tip should be sharp, free from kinks, nicks, and hooks when examined under magnification 2.5 times. Puncture needle and
Tensile axial tension of 10 N is applied for 15 s at the joint of the needle shank and should not be broken or loosened.
6.3.4 Fitting length of puncture needle and catheter
The end of the catheter should be tapered to facilitate insertion into the skin and fit tightly with the puncture needle. When the puncture needle is fully inserted into the catheter assembly,
The tip of the catheter should neither exceed the root of the bevel of the needle tip, nor should it leave it more than 1 mm (see dimension a in Figure 2).
6.4 Separators (if any)
6.4.1 Self-sealing
When the plug of the separator is disconnected from the socket, the socket shall be self-sealing. When tested according to A.2.2, there shall be no leakage.
6.4.2 Ease of Connectivity
The plug of the separator should be easily inserted into the socket, and the connected separator should be easy to separate.
6.4.3 Locking device
The separator shall have a locking device. When tested in accordance with A.2.3, the locked assembly shall be capable of withstanding 10 N axial static pull for 15 s without breaking.
6.5 Paste the tape (if any)
6.5.1 release paper/separator
The adhesive tape should have a release paper/isolation film to protect the adhesive surface. The puncture component's adhesive tape should be designed to facilitate puncture.
6.5.2 Peel strength of integrated adhesive tape
When tested in accordance with A.3.1 and A.3.2, the maximum force required for an integral adhesive tape shall not be less than 2.0N.
6.5.3 Peel strength of separate adhesive tape
When tested in accordance with A.3.1 and A.3.3, the maximum force required for each 1cm width of the separate adhesive tape shall not be less than 0.5N.
6.5.4 Water vapor permeability
If the adhesive tape is transparent to water vapor, it should be able to provide the tape material as required in accordance with 6.2 of YY/T 0148-2006.
evidence.
6.6 Interfaces
6.6.1 Interface connected to insulin reservoir If it is designed as a needle-free interface, it should be an inner cone that meets the requirements of GB/T 1962.2.
Lock the connector;
6.6.2 Interfaces connected to insulin reservoirs If the interface is designed to have a needle connection, the following requirements should be met.
a) The length of the needle tube should be sufficient to ensure penetration of the seal of the reservoir;
b) The needle handle should be a hood-type structure, which acts as a needle-proof for the puncture needle;
c) After the...
Get Quotation: Click YY/T 1291-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1291-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1291-2016: Single use subcutaneous infusion sets for use with insulin pump
YY/T 1291-2016
Single use subcutaneous infusion sets for use with insulin pump
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Disposable insulin pump subcutaneous infusion device
Published on.2016-03-23
2017-01-01 Implementation
The State Food and Drug Administration issued
Directory
Preface III
Introduction IV
1 Range 1
2 Normative references 1
3 Terms and Definitions 1
4 Structure and composition 2
5 Material 3
6 Physical Requirements 3
6.1 Line 3
6.1.1 Appearance 3
6.1.2 Transparency 3
6.1.3 Length 3
6.2 Hypodermic needle (if any) 3
6.2.1 Needle 3
6.2.2 Needle length 3
6.2.3 Needle bend direction and angle 3
6.2.4 Tip 4
6.2.5 needle handle connection firmness 4
6.3 Subcutaneous trocar (if any) 4
6.3.1 Conduit length 4
6.3.2 Catheter and catheter base connection firmness 4
6.3.3 Puncture needle 4
6.3.4 Fitting length of puncture needle and catheter 4
6.4 Separators (if any) 4
6.4.1 Self-sealing 4
6.4.2 Easy Connectivity 4
6.4.3 Locking device 4
6.5 Adhesive tape (if any) 4
6.5.1 release paper/separator 4
6.5.2 Peeling Strength of Integrated Adhesive Tape 4
6.5.3 Separating adhesive tape peel strength 5
6.5.4 Water vapor permeability 5
6.6 Interface 5
6.7 Pipe Connection Strength 5
6.8 Corrosion resistance 5
6.9 Particle Pollution 5
6.10 Leak 5
6.11 Smoothness 5
6.12 Stability 5
6.12.1 resistance to external interference 5
6.12.2 Flex resistance 5
6.13 pill volume 6
6.14 protective cover 6
7 Chemical properties 6
7.1 Preparation of test solution 6
7.2 Reducing substances 6
7.3 Metal Ions 6
7.4 pH Titrations 6
7.5 evaporation residue 6
7.6 UV absorbance 6
8 Biological properties 6
8.1 Biocompatibility 6
8.2 Sterile 7
8.3 Bacterial endotoxin 7
9 Type Inspection 7
10 Signs 7
10.1 Single Pack 7
10.2 Attached Document 7
10.3 Packing 8
10.4 Transport Package 8
11 Packing 9
Appendix A (Normative) Physical Test 10
Appendix B (Informative) Design Guidelines for Subcutaneous Infusion Sets 13
Reference 14
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This standard is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
This standard is mainly drafted by. Shandong Provincial Medical Device Product Quality Inspection Center.
Participated in the drafting of this standard. Zhengzhou Ruiyu Technology Co., Ltd., Medtronic (Shanghai) Management Co., Ltd.
The main drafters of this standard. Liu Lili, Yao Xiujun, Wang Yongxin, Wang Jinhong, and Jia Yufei.
introduction
The disposable infusion pump for disposable insulin pump is generally composed of an interface, a pipeline, and a puncturing component, and is connected with a drug reservoir contained in an insulin pump.
Then, the micro-computer controls the injection of exogenous insulin on time, quantitatively and accurately to the user's skin, keeping the blood sugar level for 24 hours stable.
Control the purpose of diabetes.
This standard will be divided into subcutaneous needle and subcutaneous trocar.
This standard does not classify subcutaneous infusion sets for disposable insulin pumps.
This standard provides design guidelines in the form of informative annexes (Appendix B).
This standard stipulates the requirements for single-use subcutaneous infusion sets for insulin pumps and provides a unified evaluation of disposable insulin pumps.
Use the performance indicators and test methods for the stability of subcutaneous infusion sets.
Disposable insulin pump subcutaneous infusion device
1 Scope
This standard specifies the requirements for subcutaneous infusion sets for insulin pumps (referred to as "subcutaneous infusion sets") consisting of interfaces, tubing and piercing components.
This product is a single use sterile product.
This standard does not include the requirements for insulin-filled devices (eg, drug reservoirs, pre-filled cartridges) in insulin pumps.
This standard does not address the accuracy requirements for flow control when a subcutaneous infusion set is fitted with an insulin pump.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 1962.2 Syringes, injection needles and other medical devices 6% (Luer) conical joints Part 2. Locking joints
(GB/T 1962.2-2001, ISO 594-2.1998, IDT)
GB 8368-2005 Single-use infusion set for gravity infusion (ISO 8536-4.2004, MOD)
GB 9706.27 Medical electrical equipment - Part 22-24. Particular requirements for the safety of infusion pumps and infusion controllers (GB 9706.27-
2005, IEC 60601-2-24.1998, IDT)
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 14233.2-2005 Methods for the examination of medical infusions, blood transfusions and syringes - Part 2. Biological methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Evaluation and testing in risk management process (GB/T 16886.1-
2011, ISO 10993-1.2009, IDT)
GB 18457 Stainless steel needles for medical devices (GB 18457-2001, ISO 9626.1991, IDT)
YY/T 0148-2006 Medical Tape General Requirements
YY 0285.1-2004 Disposable sterile endovascular catheters Part 1. General requirements (ISO 10555-1.1995, IDT)
YY 0285.5-2004 Disposable sterile intravascular catheters Part 5. Trocar peripheral catheters (ISO 10555-5.1996,
IDT)
YY/T 0466.1 Medical Devices Symbols for Medical Devices Signs, Labels, and Information Provided (YY/T 0466.1-2009,
ISO 15223-1.2007, IDT)
YY/T 0615.1 Requirements for Labeling “Aseptic” Medical Devices Part 1. Requirements for Final Sterilization Medical Devices
ISO 11607-1.2006 Packaging for terminally sterilized medical devices Part 1. Requirements for materials, sterile barrier systems, and packaging systems 1)
1) The Chinese standard GB/T 19633.1 equivalent to ISO 11607-1.2006 is currently in the approval stage.
3 Terms and Definitions
The following terms and definitions apply to this document.
3.1
Piercing component introducerneedleunit
Subcutaneous infusion device for subcutaneous puncture components, usually subcutaneous needle (or subcutaneous trocar), needle handle (or catheter base), fixed paste
Tape composition.
3.2
Hypodermic needlenee
Rigid tube with cutting edge on one end for subcutaneous administration, typically with oblique insertion and in-line insertion
3.3
Subcutaneous trocar hypodermicneedleandcatheterassembly
An assembly consisting of a metal piercing needle and a catheter over it. After the subcutaneous trocar is punctured into the skin, the metal puncture needle is pulled out.
Placed subcutaneously for subcutaneous administration.
3.4
Separator segregator
An assembly on the tubing near the piercing assembly for disconnecting and connecting the piercing assembly from the insulin pump.
Note. The purpose of the separator is to facilitate the use of people who need to temporarily remove the insulin pump.
3.5
Interface connector
The connection between the subcutaneous infusion set and the reservoir of the insulin pump.
3.6
Fixed surface adhesivesurface
Attach the adhesive tape to the adhesive side.
3.7
Fixed segment fixedsegment
A subcutaneous infusion set with a separator is from the piercing assembly to the section between the outlets of the separator.
3.8
Movable segment removesegment
The subcutaneous infusion set with the separator is from the separator head to the interface between the connectors.
4 Structure and composition
The structure and composition of a typical subcutaneous infusion set are shown in Figure 1.
Explanation.
1---Paste adhesive tape for puncture assembly (integrated) a;
2 --- needle handle;
3 --- subcutaneous needle b;
4---pipeline;
5---Separator socket c;
6---plug c of the splitter;
7---Separator adhesive tape (integral) d;
8---pipeline;
9--- interface.
a can also be a separate adhesive tape provided separately;
b. The hypodermic needle may also be the structure of the trocar shown in Fig. 2;
c is an optional component;
d can also be a separate adhesive tape provided separately.
Fig.1 Schematic diagram of subcutaneous infusion set for insulin pump
Note. Figure 1 is for illustration purposes only and is not the only type specified in this standard.
Explanation.
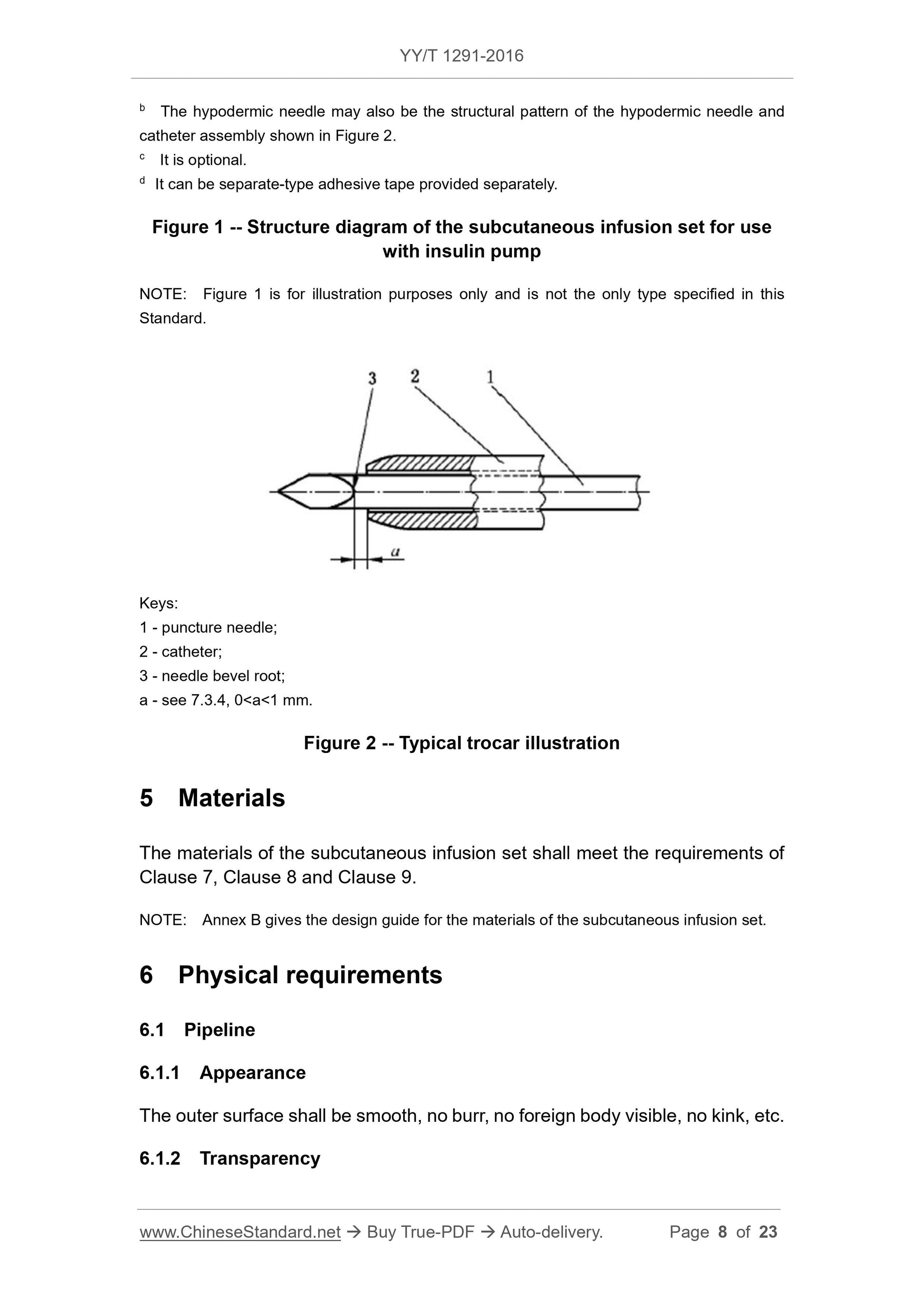
1 --- puncture needle;
2 --- conduit;
3 --- the root of the needle tip slope;
a--- see 7.3.4,0 \u003ca\u003c1mm。
Figure 2 Typical trocar illustration
5 Materials
The material of the subcutaneous infusion set should meet the requirements specified in Chapter 7, Chapter 8, and Chapter 9.
Note. Appendix B provides design guidelines for subcutaneous infusion set materials.
6 Physical requirements
6.1 Pipeline
6.1.1 Appearance
The outer surface should be smooth, free from burrs, visible to the naked eye, and free from kinks.
6.1.2 Transparency
It should be transparent or sufficiently transparent. When tested according to A.1, it should be able to detect the gas-liquid interface.
6.1.3 Length
The length of the pipeline should not be less than 90% of the nominal value.
6.2 Hypodermic needle (if any)
6.2.1 Needle
The needle section and the wall thickness should be uniform, and the liquid passage of the needle should be clear.
6.2.2 Needle length
The effective length of the needle tube should meet the requirements of the nominal value, and the tolerance is ±1mm.
Note. The effective length of the needle is the length of the first bend from the needle tip to the needle. For in-line hypodermic needles, the first bend is the needle and wear
The thorn component is pasted at the junction of the tape, ie, the bending angle is close to 90°; for the oblique insertion type hypodermic needle, the first bend is exposed at the outside bending point.
Discounts.
6.2.3 Needle bend direction and angle
When the needle bending angle is visually observed parallel to the fixing surface of the needle shank and the direction of the tube (in the direction shown in Fig. 1), the needle tube and the needle
The angle on the pipe side of the shank should be equal to or greater than 90°.
6.2.4 Tip
With normal vision or corrected vision, the needle tip should be sharp, free from kinks, nicks, and hooks when examined under magnification 2.5 times.
6.2.5 Fastness of needle connection
The static tensile force of 10 N applied for 10 seconds between the hypodermic needle and the handle is not to be broken or loosened.
6.3 Subcutaneous trocar (if any)
6.3.1 Catheter length
The effective length of the catheter (the length from the tip of the catheter to the end of the catheter) should be expressed in millimeters. The effective length of the catheter should meet the nominal value
The tolerance is ±1mm.
6.3.2 The connection between catheter and catheter base
The connection between the catheter and the base of the catheter should be able to withstand a static axial tension of 3 N for 15 s and there should be no loosening or separation of the connection.
6.3.3 Needle
With normal vision or corrected vision, the needle tip should be sharp, free from kinks, nicks, and hooks when examined under magnification 2.5 times. Puncture needle and
Tensile axial tension of 10 N is applied for 15 s at the joint of the needle shank and should not be broken or loosened.
6.3.4 Fitting length of puncture needle and catheter
The end of the catheter should be tapered to facilitate insertion into the skin and fit tightly with the puncture needle. When the puncture needle is fully inserted into the catheter assembly,
The tip of the catheter should neither exceed the root of the bevel of the needle tip, nor should it leave it more than 1 mm (see dimension a in Figure 2).
6.4 Separators (if any)
6.4.1 Self-sealing
When the plug of the separator is disconnected from the socket, the socket shall be self-sealing. When tested according to A.2.2, there shall be no leakage.
6.4.2 Ease of Connectivity
The plug of the separator should be easily inserted into the socket, and the connected separator should be easy to separate.
6.4.3 Locking device
The separator shall have a locking device. When tested in accordance with A.2.3, the locked assembly shall be capable of withstanding 10 N axial static pull for 15 s without breaking.
6.5 Paste the tape (if any)
6.5.1 release paper/separator
The adhesive tape should have a release paper/isolation film to protect the adhesive surface. The puncture component's adhesive tape should be designed to facilitate puncture.
6.5.2 Peel strength of integrated adhesive tape
When tested in accordance with A.3.1 and A.3.2, the maximum force required for an integral adhesive tape shall not be less than 2.0N.
6.5.3 Peel strength of separate adhesive tape
When tested in accordance with A.3.1 and A.3.3, the maximum force required for each 1cm width of the separate adhesive tape shall not be less than 0.5N.
6.5.4 Water vapor permeability
If the adhesive tape is transparent to water vapor, it should be able to provide the tape material as required in accordance with 6.2 of YY/T 0148-2006.
evidence.
6.6 Interfaces
6.6.1 Interface connected to insulin reservoir If it is designed as a needle-free interface, it should be an inner cone that meets the requirements of GB/T 1962.2.
Lock the connector;
6.6.2 Interfaces connected to insulin reservoirs If the interface is designed to have a needle connection, the following requirements should be met.
a) The length of the needle tube should be sufficient to ensure penetration of the seal of the reservoir;
b) The needle handle should be a hood-type structure, which acts as a needle-proof for the puncture needle;
c) After the...
Share