1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1470-2016 English PDF (YY/T1470-2016)
YY/T 1470-2016 English PDF (YY/T1470-2016)
Regular price
$130.00 USD
Regular price
Sale price
$130.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1470-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1470-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1470-2016: Disposable cut of the umbilical cord
YY/T 1470-2016
Disposable cut of the umbilical cord
ICS 11.040.30
C36
People's Republic of China Pharmaceutical Industry Standard
One-time use of umbilical cord scissors (cutting)
Published on.2016-01-26
2017-01-01 Implementation
The State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. Publication of this document
The agency does not assume responsibility for identifying these patents.
This standard is proposed and managed by the National Standardization Technical Committee for Family Planning Devices (SAC/TC169).
This standard was drafted by. Changzhou Sanlian Xinghai Medical Device Manufacturing Co., Ltd., Shanghai Medical Device Testing and Shanghai Heng Instrument Factory
Limited company.
The main drafters of this standard are. Tian Runting, Yao Tianping, Weng Binghao, Zou Bing, and Jiang Songbo.
One-time use of umbilical cord scissors (cutting)
1 Scope
This standard specifies the structural type, requirements, test methods, inspection rules, signs and instructions for the use of single-use umbilical cord shears.
Books, packaging, transportation and storage.
This standard applies to a one-time use of umbilical cord cutting (cutting) breakers. This product is used to clamp and cut off the umbilical cord of neonates during delivery of obstetrics.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 191 packaging, storage and transportation logo
GB/T 2828.1 Count Sampling Inspection Procedures Part 1. Batch Inspection Sampling Plans Retrieved by Acceptance Quality Limit (AQL)
GB/T 2829 Periodic Inspection Count Sampling Procedures and Tables (Applicable to Inspection of Process Stability)
GB 3280-2015 Stainless Steel Cold-rolled Steel Sheet and Strip
GB/T 4340.1-2009 Vickers hardness test for metallic materials - Part 1. Test method
GB/T 9969 Instruction Manual for Industrial Products
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB/T 16886.5-2003 Biological evaluation of medical devices Part 5. In vitro cytotoxicity test
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10. Stimulation and delayed type hypersensitivity tests
YY/T 0031-2008 Silicone rubber pipelines and elastic parts for transfusion and blood transfusion
YY/T 0149-2006 Test Method for Corrosion Resistance of Stainless Steel Medical Devices
YY/T 0171 Surgical Instrument Packaging, Signs, and Instructions for Use
Pharmacopoeia of the People's Republic of China (2015 version)
3 Structure
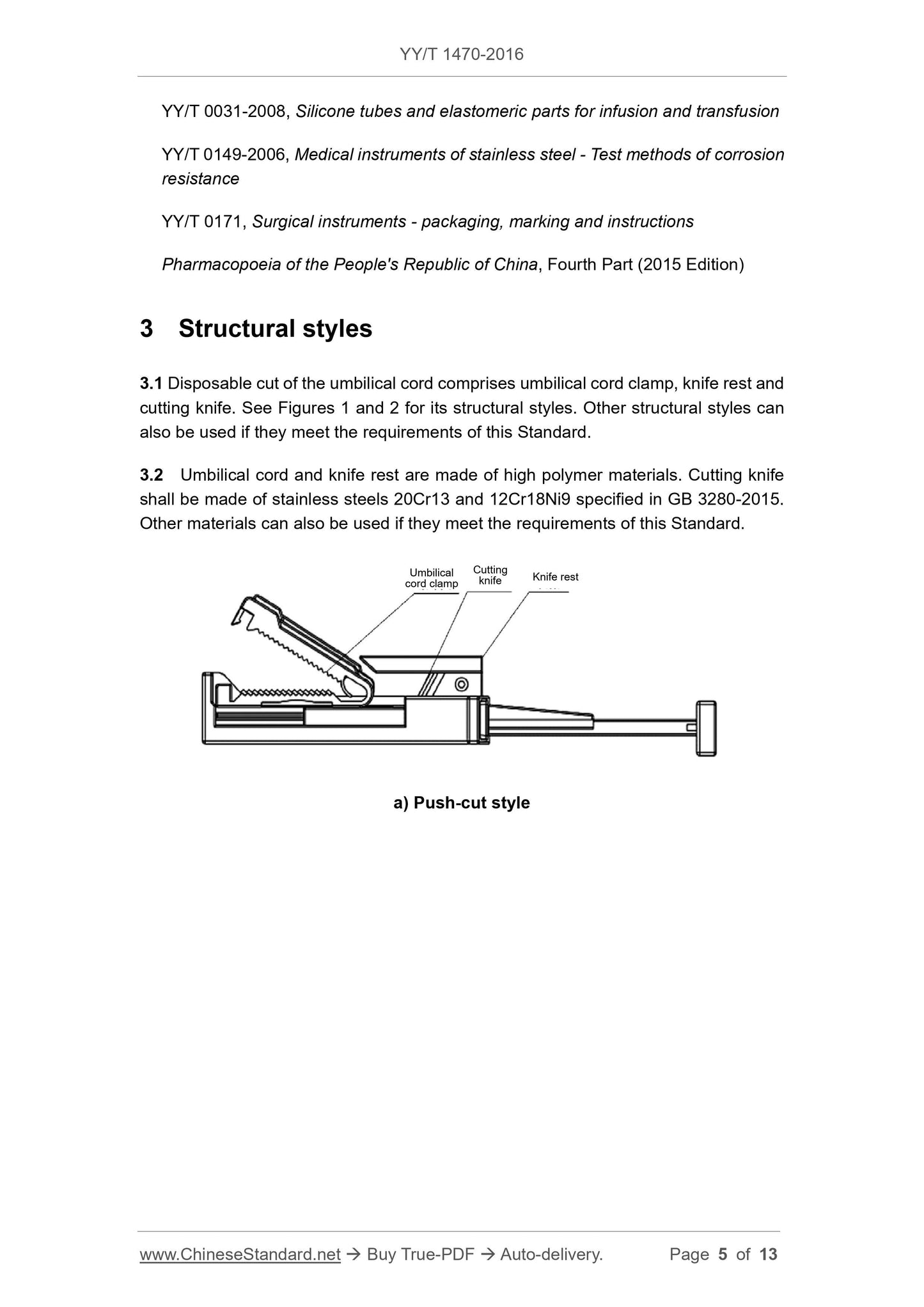
3.1 One-time use of umbilical cord scissors (cutting) The disconnector is composed of an umbilical cord clamp, a knife holder and a cutting knife. Its structure type is shown in Fig. 1 and Fig. 2 . If you can comply with this
The requirements of the standard can also be other structural types.
3.2 The umbilical cord clamp and knife holder are made of polymer material. Cutters shall comply with 20Cr13 and 12Cr18Ni9 stainless steels in GB 3280-2015
Made of steel material. Other materials may also be used if they meet the requirements of this standard.
a) Push type
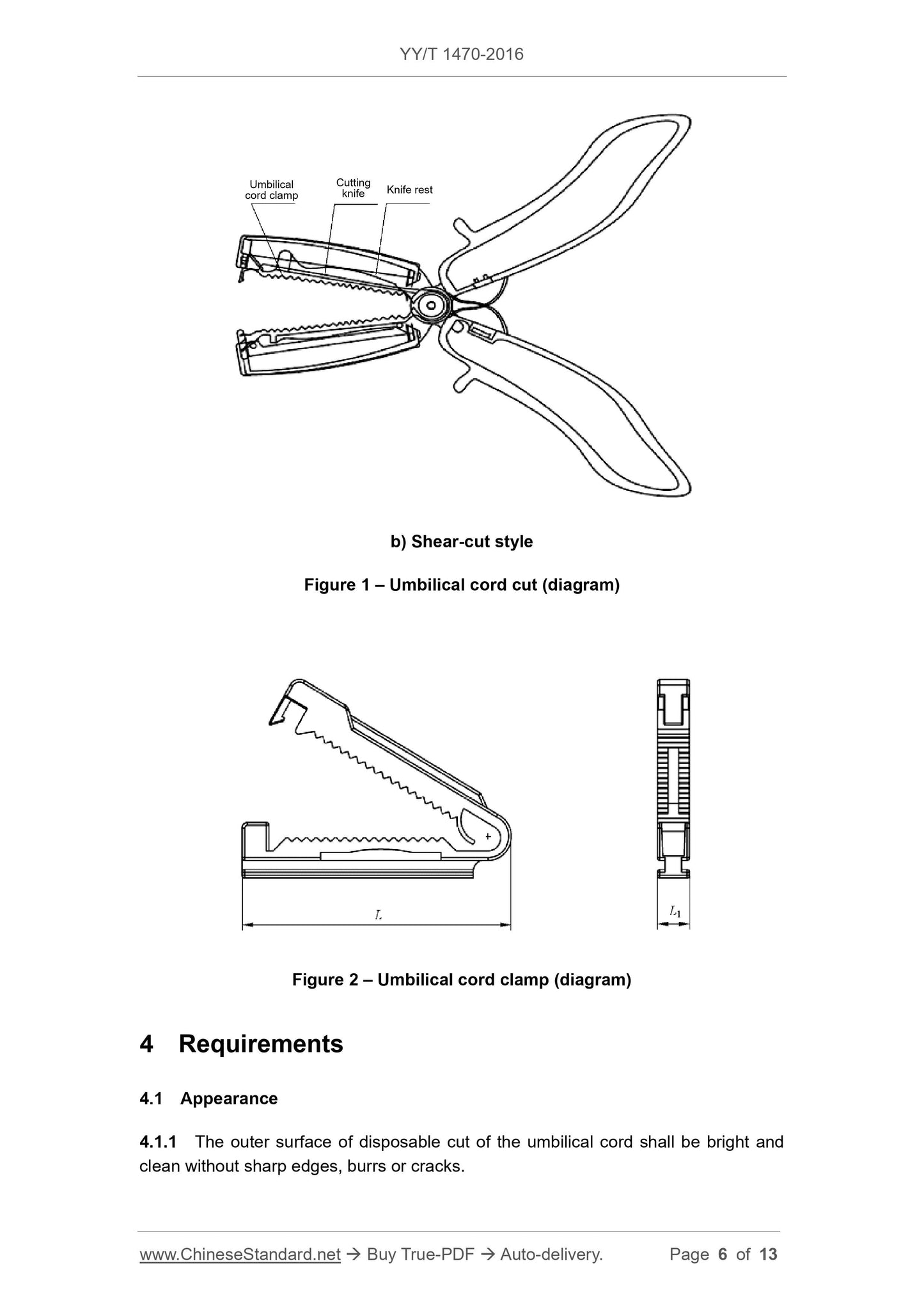
b) Shear
Figure 1 Umbilical cord cutter (cutting) (Schematic)
Figure 2 Umbilical cord clamp (schematic diagram)
4 Requirements
4.1 Appearance
4.1.1 The outer surface of the disposable umbilical cord cutter (cutting) breaker should be clean and free of sharp edges, burrs and cracks.
4.1.2 The surface of the cutter should be smooth, free from oil, rust, and missing edges.
4.2 Size
The umbilical cord clamp size should meet the requirements of Table 1.
Table 1 The basic dimensions and tolerances are in millimeters
Umbilical cord size tolerance
L 50~60 ±2
L1 5~7 ±0.5
4.3 cutting blade
4.3.1 Surface roughness
The surface roughness of the cutting blade should not exceed 0.8 μm.
4.3.2 Hardness
The hardness of the cutting blade should not be less than 377HV0.2.
4.3.3 Corrosion resistance
The cutting blade should have good corrosion resistance and should meet the requirements of class b of the boiling water test method in YY/T 0149-2006.
4.4 Using performance
4.4.1 cut off performance
One-time use of umbilical cord scissors (cutting) breakers should have good shear (cutting) breaking performance.
4.4.2 Clamping performance
One-time use of umbilical cord scissors (cutting) breakers should have good gripping performance and should withstand a static load of 15 N after clamping.
4.4.3 Matching performance
One-time use of the umbilical cord shear (cutting) breaker shall have good fitting performance, and there shall be no phenomenon of shedding or blocking during shearing (cutting).
4.5 Sterility
The sterilized sterilization process has been confirmed, the sterilized disposable umbilical cord cutter (cutting) should be sterile.
4.6 Ethylene Oxide Residue
If the disposable umbilical cord cutter is used for ethylene oxide sterilization, the residual amount of ethylene oxide should not exceed 10 μg/g.
4.7 Biological Evaluation
4.7.1 In vitro cytotoxicity
The cytotoxicity of a single-use umbilical cord scissors (shear) breaker should be no greater than 2 levels.
4.7.2 Delayed hypersensitivity
One-time use of umbilical cord scissors (shear) breakers should be without delayed type hypersensitivity.
4.7.3 Intradermal reaction test
One-time use of umbilical cord scissors (cutting) test specimens and solvent control, the difference between the average score should not exceed 1.0.
5 test methods
5.1 Appearance
5.1.1 Inspection with normal or corrected visual acuity and hand rubbing shall comply with the provisions of 4.1.1.
5.1.2 With normal or corrected visual acuity, the requirements of 4.1.2 shall be met.
5.2 Size
Measured with universal gauges, shall comply with the provisions of 4.2.
5.3 cutting blade
5.3.1 Surface roughness
The measurement by surface roughness comparison specimens or electrical measurements shall be measured by electrical measurements during arbitration and shall comply with the requirements of 4.3.1.
5.3.2 Hardness
Test according to the method specified in GB/T 4340.1-2009, shall meet the requirements of 4.3.2.
5.3.3 Corrosion resistance
According to YY/T 0149-2006 boiling water test method test, should meet the requirements of 4.3.3.
5.4 Using Performance
5.4.1 cut off performance
Using the 5mm×7mm infusion tube shown in Table 1 of YY/T 0031-2008, the imitating shear (cutting) action, the transfusion tube is flat, no adhesion, and cutting
The knife shall not be wound or chipped, and shall comply with the provisions of 4.4.1.
5.4.2 Clamping performance
The umbilical cord clamp is used to clamp the infusion tube specified in 5.4.1, and the umbilical cord clamp with the infusion tube clamped is fixed with a clamp, and the other end of the tube is added with 15N static.
The load, which lasts 10s, shall comply with the provisions of 4.4.2.
5.4.3 Matching performance
Imitation shearing (cutting) breaking action, clamping the umbilical cord clamp before the infusion tube specified in 5.4.1 does not fall off, clamping the infusion tube does not block after shearing, should be consistent with
The provisions of 4.4.3.
5.5 Sterility
Inspection according to the 1101 sterility inspection method of the “Pharmacopoeia of the People's Republic of China (Part IV)” (2015 Edition) (2015 version) shall comply with the provisions of 4.5.
5.6 Ethylene Oxide Residue
According to GB/T 14233.1-2008 ethylene oxide residue test method for testing, shall comply with the provisions of 4.6.
5.7 Biological Evaluation
5.7.1 In vitro cytotoxicity
According to GB/T 16886.5-2003 8.2 (extract liquid test) prescribed method, shall comply with the provisions of 4.7.1.
5.7.2 Delayed hypersensitivity
According to the method specified in 7.4 (maximum dose test) in GB/T 16886.10-2005, it shall comply with the requirements of 4.7.2.
5.7.3 Intradermal reaction test
According to the method specified in Appendix B.2 (intradermal reaction test) in GB/T 16886.10-2005, it shall comply with the provisions of 4.7.3.
6 Inspection Rules
6.1 Acceptance
A one-time use of the umbilical cord cutting (cutting) disconnector should be inspected by the manufacturer's quality inspection department, and acceptance can be submitted only after passing the inspection.
6.2 Inspection Methods
One-time use of umbilical cord scissors (cutting) disconnectors shall be submitted in batches. The inspection shall be divided into batch-by-batch inspection (factory inspection) and periodic inspection (type inspection
Test).
6.3 batch inspection
6.3.1 batch by batch test according to GB/T 2828.1 regulations.
6.3.2 Sampling plan adopts one-time sampling. The strictness of the sampling plan starts from the normal inspection sampling plan, and its unqualified classification, inspection group,
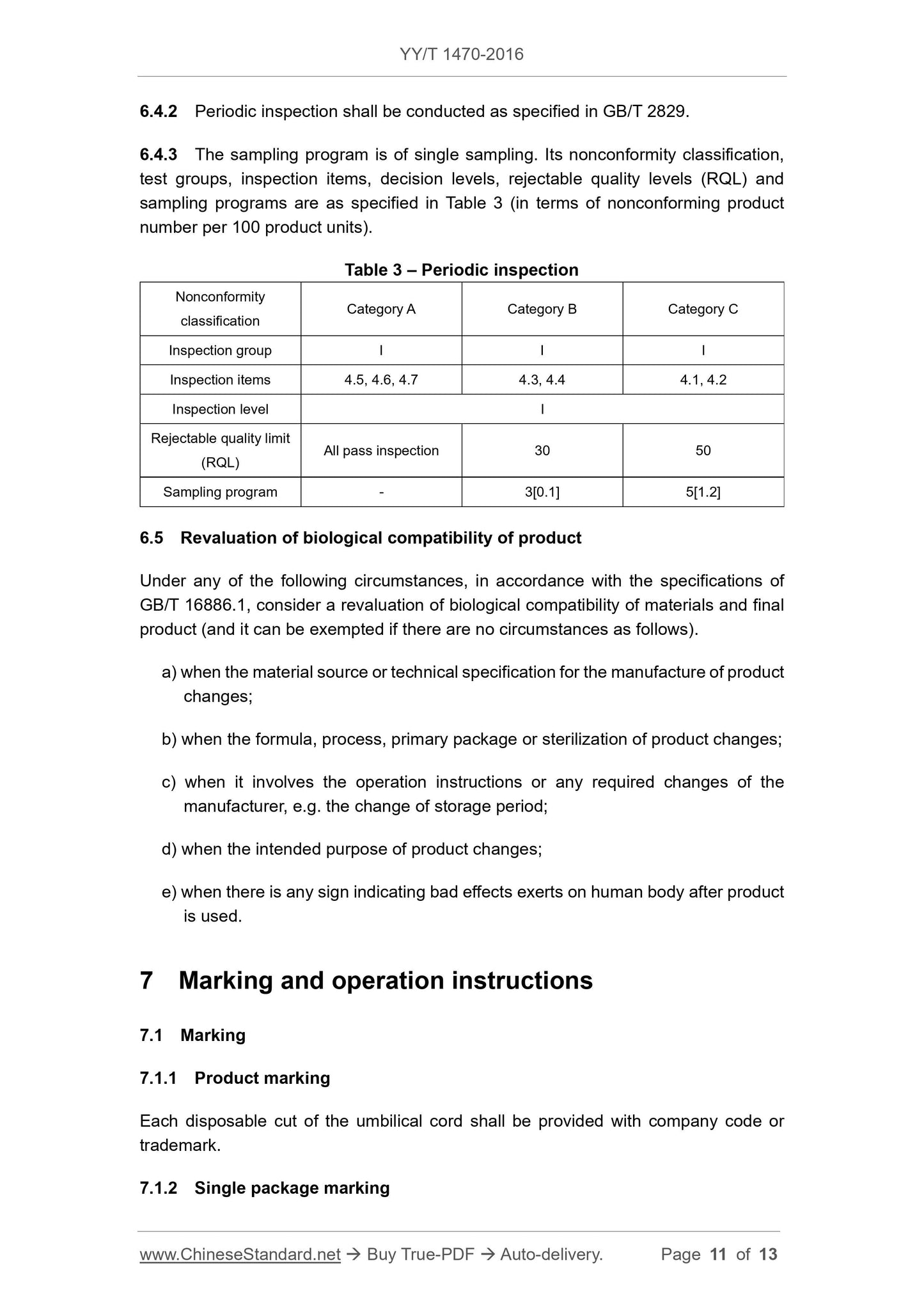
Inspection items, inspection levels, and acceptance quality limits (AQL) are in accordance with Table 2 (calculated on the basis of the number of nonconforming products per 100 units of product).
Table 2 batch inspection
Failure Category A Class B Class C
Inspection Group I I I
Inspection items 4.5, 4.6 4.3, 4.4 4.1, 4.2
Inspection Level - S-2 S-3
Acceptance Quality Limit (AQL) All Qualified 4.0 6.5
6.4 periodic inspection
6.4.1 In the following cases, periodic inspections should be conducted.
a) Before the new product is put into operation (including the conversion of old products into production);
b) When it is re-launched at intervals of more than one year;
c) when there is a major change in product design, process or material;
d) Continuously produced products once every two years;
e) When requested by the National Quality Supervision and Inspection Department.
6.4.2 The periodic inspection shall be conducted in accordance with the provisions of GB/T 2829.
6.4.3 The periodic inspection adopts a sampling plan. The unqualified classification, test group, inspection item, discrimination level, and unqualified quality level
(RQL) and sampling plans are as specified in Table 3 (calculated on the basis of the number of nonconforming products per hundred units of product).
Table 3 periodic inspection
Failure Category A Class B Class C
Test group I I I
Inspection items 4.5, 4.6, 4.7 4.3, 4.4 4.1, 4.2
Level of discrimination I
Unqualified Quality Level (RQL) All Qualified 30 50
Sampling plan - 3 [0.1] 5 [1.2]
6.5 Reassessing Product Biocompatibility
In any of the following cases, reassessment of biological evaluation of materials or final products should be considered as specified in GB/T 16886.1.
(Can be exempted from the following conditions).
a) When the source or technical specification of the material used to manufacture the product changes;
b) When product formulation, process, primary packaging or sterilization change;
c) any change in manufacturer's instructions or requirements involving storage, such as when the storage period changes;
d) When the intended use of the product changes;
e) There are indications that the product will be defective when applied to the human body.
7 Signs and Instruction Manual
7.1 Logo
7.1.1 Product Logo
Every single-use umbilical cord cutter (cutting device) should have a company code or trademark.
7.1.2 Single Packing Mark
The single-use umbilical cord cutter (cutting) markers on the single package should be clear and should have the following signs.
a) manufacturer's name or trademark;
b) product name and product specification;
c) product registration certificate number;
d) production lot number or date;
e) the words "sterile" and/or sterile graphic symbols, "destroyed after use", and "breakage of prohibited packaging" are used;
f) Sterilization method and expiration date.
7.1.3 Packaging Symbol
a) manufacturer's name or trademark, address;
b) product name and product specification;
c) product registration certificate number;
d) "One-time use" words or graphic symbols;
e) Sterilization mark;
f) the number of products;
g) Expiration date.
7.1.4 box mark
a) company name, trademark, address, telephone number;
b) product name, quantity, quality;
c) product registration certificate number or standard number;
d) sterilization batch number;
e) gross weight, volume (length × width × height);
f) The signs related to storage and transportation such as "afraid of the sun", "afraid of rain" and "be careful of light discharge" should conform to the provisions of GB/T 191.
7.2 Instruction Manual
The instruction manual should meet the requirements of GB/T 9969 and YY/T 0171, and indicate the following.
a) company name, address, telephone number;
b) product name, registered trademark;
c) product registration number, registered product standard number, and medical device manufacturing enterprise license number;
d) Product performance, manufacturing materials, main structure;
e) "Disposable", "non-reusable", "not allowed to be used when the minimum package is damaged", sterilization method and use validity period or
Expiration date and eye-catching "sterile";
f) the scope of application of the product, contraindications, related precautions, and other matters requiring warning or prompting;
g) Ensure the correct and safe use of the product.
8 Packaging, Transport and Storage
8.1 Packaging
8.1.1 The single package shall be packed in a plastic bag and sealed with a disposable umbilical cord cutter. Small packages should be kept dry and clean.
8.1.2 The one-time use of the umbilical cord cutting (cutting) breaker shall be securely wrapped. There shall be instructions for use and product certification in the outer packaging.
8.2 Transportation
Packing and shipping requirements are in accordance with the contract.
8.3 Stor...
Get Quotation: Click YY/T 1470-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1470-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1470-2016: Disposable cut of the umbilical cord
YY/T 1470-2016
Disposable cut of the umbilical cord
ICS 11.040.30
C36
People's Republic of China Pharmaceutical Industry Standard
One-time use of umbilical cord scissors (cutting)
Published on.2016-01-26
2017-01-01 Implementation
The State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. Publication of this document
The agency does not assume responsibility for identifying these patents.
This standard is proposed and managed by the National Standardization Technical Committee for Family Planning Devices (SAC/TC169).
This standard was drafted by. Changzhou Sanlian Xinghai Medical Device Manufacturing Co., Ltd., Shanghai Medical Device Testing and Shanghai Heng Instrument Factory
Limited company.
The main drafters of this standard are. Tian Runting, Yao Tianping, Weng Binghao, Zou Bing, and Jiang Songbo.
One-time use of umbilical cord scissors (cutting)
1 Scope
This standard specifies the structural type, requirements, test methods, inspection rules, signs and instructions for the use of single-use umbilical cord shears.
Books, packaging, transportation and storage.
This standard applies to a one-time use of umbilical cord cutting (cutting) breakers. This product is used to clamp and cut off the umbilical cord of neonates during delivery of obstetrics.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 191 packaging, storage and transportation logo
GB/T 2828.1 Count Sampling Inspection Procedures Part 1. Batch Inspection Sampling Plans Retrieved by Acceptance Quality Limit (AQL)
GB/T 2829 Periodic Inspection Count Sampling Procedures and Tables (Applicable to Inspection of Process Stability)
GB 3280-2015 Stainless Steel Cold-rolled Steel Sheet and Strip
GB/T 4340.1-2009 Vickers hardness test for metallic materials - Part 1. Test method
GB/T 9969 Instruction Manual for Industrial Products
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB/T 16886.5-2003 Biological evaluation of medical devices Part 5. In vitro cytotoxicity test
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10. Stimulation and delayed type hypersensitivity tests
YY/T 0031-2008 Silicone rubber pipelines and elastic parts for transfusion and blood transfusion
YY/T 0149-2006 Test Method for Corrosion Resistance of Stainless Steel Medical Devices
YY/T 0171 Surgical Instrument Packaging, Signs, and Instructions for Use
Pharmacopoeia of the People's Republic of China (2015 version)
3 Structure
3.1 One-time use of umbilical cord scissors (cutting) The disconnector is composed of an umbilical cord clamp, a knife holder and a cutting knife. Its structure type is shown in Fig. 1 and Fig. 2 . If you can comply with this
The requirements of the standard can also be other structural types.
3.2 The umbilical cord clamp and knife holder are made of polymer material. Cutters shall comply with 20Cr13 and 12Cr18Ni9 stainless steels in GB 3280-2015
Made of steel material. Other materials may also be used if they meet the requirements of this standard.
a) Push type
b) Shear
Figure 1 Umbilical cord cutter (cutting) (Schematic)
Figure 2 Umbilical cord clamp (schematic diagram)
4 Requirements
4.1 Appearance
4.1.1 The outer surface of the disposable umbilical cord cutter (cutting) breaker should be clean and free of sharp edges, burrs and cracks.
4.1.2 The surface of the cutter should be smooth, free from oil, rust, and missing edges.
4.2 Size
The umbilical cord clamp size should meet the requirements of Table 1.
Table 1 The basic dimensions and tolerances are in millimeters
Umbilical cord size tolerance
L 50~60 ±2
L1 5~7 ±0.5
4.3 cutting blade
4.3.1 Surface roughness
The surface roughness of the cutting blade should not exceed 0.8 μm.
4.3.2 Hardness
The hardness of the cutting blade should not be less than 377HV0.2.
4.3.3 Corrosion resistance
The cutting blade should have good corrosion resistance and should meet the requirements of class b of the boiling water test method in YY/T 0149-2006.
4.4 Using performance
4.4.1 cut off performance
One-time use of umbilical cord scissors (cutting) breakers should have good shear (cutting) breaking performance.
4.4.2 Clamping performance
One-time use of umbilical cord scissors (cutting) breakers should have good gripping performance and should withstand a static load of 15 N after clamping.
4.4.3 Matching performance
One-time use of the umbilical cord shear (cutting) breaker shall have good fitting performance, and there shall be no phenomenon of shedding or blocking during shearing (cutting).
4.5 Sterility
The sterilized sterilization process has been confirmed, the sterilized disposable umbilical cord cutter (cutting) should be sterile.
4.6 Ethylene Oxide Residue
If the disposable umbilical cord cutter is used for ethylene oxide sterilization, the residual amount of ethylene oxide should not exceed 10 μg/g.
4.7 Biological Evaluation
4.7.1 In vitro cytotoxicity
The cytotoxicity of a single-use umbilical cord scissors (shear) breaker should be no greater than 2 levels.
4.7.2 Delayed hypersensitivity
One-time use of umbilical cord scissors (shear) breakers should be without delayed type hypersensitivity.
4.7.3 Intradermal reaction test
One-time use of umbilical cord scissors (cutting) test specimens and solvent control, the difference between the average score should not exceed 1.0.
5 test methods
5.1 Appearance
5.1.1 Inspection with normal or corrected visual acuity and hand rubbing shall comply with the provisions of 4.1.1.
5.1.2 With normal or corrected visual acuity, the requirements of 4.1.2 shall be met.
5.2 Size
Measured with universal gauges, shall comply with the provisions of 4.2.
5.3 cutting blade
5.3.1 Surface roughness
The measurement by surface roughness comparison specimens or electrical measurements shall be measured by electrical measurements during arbitration and shall comply with the requirements of 4.3.1.
5.3.2 Hardness
Test according to the method specified in GB/T 4340.1-2009, shall meet the requirements of 4.3.2.
5.3.3 Corrosion resistance
According to YY/T 0149-2006 boiling water test method test, should meet the requirements of 4.3.3.
5.4 Using Performance
5.4.1 cut off performance
Using the 5mm×7mm infusion tube shown in Table 1 of YY/T 0031-2008, the imitating shear (cutting) action, the transfusion tube is flat, no adhesion, and cutting
The knife shall not be wound or chipped, and shall comply with the provisions of 4.4.1.
5.4.2 Clamping performance
The umbilical cord clamp is used to clamp the infusion tube specified in 5.4.1, and the umbilical cord clamp with the infusion tube clamped is fixed with a clamp, and the other end of the tube is added with 15N static.
The load, which lasts 10s, shall comply with the provisions of 4.4.2.
5.4.3 Matching performance
Imitation shearing (cutting) breaking action, clamping the umbilical cord clamp before the infusion tube specified in 5.4.1 does not fall off, clamping the infusion tube does not block after shearing, should be consistent with
The provisions of 4.4.3.
5.5 Sterility
Inspection according to the 1101 sterility inspection method of the “Pharmacopoeia of the People's Republic of China (Part IV)” (2015 Edition) (2015 version) shall comply with the provisions of 4.5.
5.6 Ethylene Oxide Residue
According to GB/T 14233.1-2008 ethylene oxide residue test method for testing, shall comply with the provisions of 4.6.
5.7 Biological Evaluation
5.7.1 In vitro cytotoxicity
According to GB/T 16886.5-2003 8.2 (extract liquid test) prescribed method, shall comply with the provisions of 4.7.1.
5.7.2 Delayed hypersensitivity
According to the method specified in 7.4 (maximum dose test) in GB/T 16886.10-2005, it shall comply with the requirements of 4.7.2.
5.7.3 Intradermal reaction test
According to the method specified in Appendix B.2 (intradermal reaction test) in GB/T 16886.10-2005, it shall comply with the provisions of 4.7.3.
6 Inspection Rules
6.1 Acceptance
A one-time use of the umbilical cord cutting (cutting) disconnector should be inspected by the manufacturer's quality inspection department, and acceptance can be submitted only after passing the inspection.
6.2 Inspection Methods
One-time use of umbilical cord scissors (cutting) disconnectors shall be submitted in batches. The inspection shall be divided into batch-by-batch inspection (factory inspection) and periodic inspection (type inspection
Test).
6.3 batch inspection
6.3.1 batch by batch test according to GB/T 2828.1 regulations.
6.3.2 Sampling plan adopts one-time sampling. The strictness of the sampling plan starts from the normal inspection sampling plan, and its unqualified classification, inspection group,
Inspection items, inspection levels, and acceptance quality limits (AQL) are in accordance with Table 2 (calculated on the basis of the number of nonconforming products per 100 units of product).
Table 2 batch inspection
Failure Category A Class B Class C
Inspection Group I I I
Inspection items 4.5, 4.6 4.3, 4.4 4.1, 4.2
Inspection Level - S-2 S-3
Acceptance Quality Limit (AQL) All Qualified 4.0 6.5
6.4 periodic inspection
6.4.1 In the following cases, periodic inspections should be conducted.
a) Before the new product is put into operation (including the conversion of old products into production);
b) When it is re-launched at intervals of more than one year;
c) when there is a major change in product design, process or material;
d) Continuously produced products once every two years;
e) When requested by the National Quality Supervision and Inspection Department.
6.4.2 The periodic inspection shall be conducted in accordance with the provisions of GB/T 2829.
6.4.3 The periodic inspection adopts a sampling plan. The unqualified classification, test group, inspection item, discrimination level, and unqualified quality level
(RQL) and sampling plans are as specified in Table 3 (calculated on the basis of the number of nonconforming products per hundred units of product).
Table 3 periodic inspection
Failure Category A Class B Class C
Test group I I I
Inspection items 4.5, 4.6, 4.7 4.3, 4.4 4.1, 4.2
Level of discrimination I
Unqualified Quality Level (RQL) All Qualified 30 50
Sampling plan - 3 [0.1] 5 [1.2]
6.5 Reassessing Product Biocompatibility
In any of the following cases, reassessment of biological evaluation of materials or final products should be considered as specified in GB/T 16886.1.
(Can be exempted from the following conditions).
a) When the source or technical specification of the material used to manufacture the product changes;
b) When product formulation, process, primary packaging or sterilization change;
c) any change in manufacturer's instructions or requirements involving storage, such as when the storage period changes;
d) When the intended use of the product changes;
e) There are indications that the product will be defective when applied to the human body.
7 Signs and Instruction Manual
7.1 Logo
7.1.1 Product Logo
Every single-use umbilical cord cutter (cutting device) should have a company code or trademark.
7.1.2 Single Packing Mark
The single-use umbilical cord cutter (cutting) markers on the single package should be clear and should have the following signs.
a) manufacturer's name or trademark;
b) product name and product specification;
c) product registration certificate number;
d) production lot number or date;
e) the words "sterile" and/or sterile graphic symbols, "destroyed after use", and "breakage of prohibited packaging" are used;
f) Sterilization method and expiration date.
7.1.3 Packaging Symbol
a) manufacturer's name or trademark, address;
b) product name and product specification;
c) product registration certificate number;
d) "One-time use" words or graphic symbols;
e) Sterilization mark;
f) the number of products;
g) Expiration date.
7.1.4 box mark
a) company name, trademark, address, telephone number;
b) product name, quantity, quality;
c) product registration certificate number or standard number;
d) sterilization batch number;
e) gross weight, volume (length × width × height);
f) The signs related to storage and transportation such as "afraid of the sun", "afraid of rain" and "be careful of light discharge" should conform to the provisions of GB/T 191.
7.2 Instruction Manual
The instruction manual should meet the requirements of GB/T 9969 and YY/T 0171, and indicate the following.
a) company name, address, telephone number;
b) product name, registered trademark;
c) product registration number, registered product standard number, and medical device manufacturing enterprise license number;
d) Product performance, manufacturing materials, main structure;
e) "Disposable", "non-reusable", "not allowed to be used when the minimum package is damaged", sterilization method and use validity period or
Expiration date and eye-catching "sterile";
f) the scope of application of the product, contraindications, related precautions, and other matters requiring warning or prompting;
g) Ensure the correct and safe use of the product.
8 Packaging, Transport and Storage
8.1 Packaging
8.1.1 The single package shall be packed in a plastic bag and sealed with a disposable umbilical cord cutter. Small packages should be kept dry and clean.
8.1.2 The one-time use of the umbilical cord cutting (cutting) breaker shall be securely wrapped. There shall be instructions for use and product certification in the outer packaging.
8.2 Transportation
Packing and shipping requirements are in accordance with the contract.
8.3 Stor...
Share