1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1499-2016 English PDF (YY/T1499-2016)
YY/T 1499-2016 English PDF (YY/T1499-2016)
Regular price
$180.00 USD
Regular price
Sale price
$180.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1499-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1499-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1499-2016: Liquid Barrier Performance and Classification of Protective Apparel Intended for Use in Health Care Facilities
YY/T 1499-2016
Liquid barrier performance and classification of protective apparel intended for use in health care facilities
ICS 11.120
C48
People's Republic of China Pharmaceutical Industry Standard
Liquid barrier properties and classification of medical protective clothing
2016-07-29 release
2017-06-01 Implementation
Published by the State Food and Drug Administration
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some elements of this document may involve patents. The issuer of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the State Food and Drug Administration Beijing Medical Device Quality Supervision and Inspection Center.
This standard was drafted. Beijing Medical Device Inspection Institute.
The main drafters of this standard. Huang Yongfu, Yue Weihua, Jiang Hongxia, Hu Guangyong.
Liquid barrier properties and classification of medical protective clothing
1 Scope
This standard specifies the classification of liquid barrier properties of medical protective clothing and related labeling requirements.
This standard applies to protective clothing marked with liquid barrier properties or liquid bio-microbial barrier properties.
This standard does not apply to other protective equipment used by medical personnel, such as. (1) not labeled or not used for the prevention of liquid or microbial barriers
Protective equipment (such as radiation protective clothing); (2) Appliances or equipment for handling hazardous chemicals, chemotherapy drugs or hazardous waste.
This standard does not apply to medical protective clothing that protects against penetration by solid particles or solid microorganisms.
This standard does not address guidelines for the proper handling or disposal of reusable medical protective clothing by medical institutions.
2 Normative references
The following documents are essential for the application of this document. For dated references, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 4744-2013 Testing and Evaluation of Textile Waterproofing Performance
YY/T 0689-2008 Blood and body fluid protection equipment Protective clothing material Anti-blood-transmitted pathogen penetration performance test Phi-
X174 bacteriophage test method
ISO 18695-2007 Textiles-Determination of Water Penetration (Textiles-Determinationofresist-
(ancetowaterpenetration-Impactpenetrationtest)
3 terms and definitions
The following terms and definitions apply to this document.
3.1
Barrier properties
Protective clothing is able to resist the ability of liquid or phytobiotic microorganisms to penetrate.
3.2
Otherpotentialyinfectiousmaterials
OPIM
In addition to blood or body fluids, it carries blood-borne pathogens or substances related to the transmission of infectious diseases.
3.3
Criticalzone
Areas on protective clothing that are most likely to come in direct contact with blood, body fluids, and other potentially infectious substances (OPIM).
3.4
Criticalzonecomponent
The critical area contains all parts, including materials, seams and attachment points for attachments.
3.5
Reinforcement area
An area in which one or two layers of the same or different materials as the product itself are added to the protective clothing to enhance or improve the performance of the product.
3.6
Seam seam
A site where two or more pieces of material are joined together.
Note. There are many ways to form seams, including traditional needle and needle stitching, as well as bonding, welding, and false stitching.
4 Requirements
4.1 Identification requirements
4.1.1 Single package
Each piece of protective clothing shall have a permanent identification of the level of liquid barrier performance, and the classification of this performance shall be carried out in accordance with 4.2.1.
If the back panel of the protective clothing cannot meet the requirements of level 1 liquid barrier performance, each protective clothing should have "no liquid protection performance on the back"
Permanent warning sign.
Each piece of protective clothing should be labeled as medical protective clothing.
4.1.2 Outer packaging
The outer packaging of each piece of protective clothing shall have a permanent identification of the level of liquid barrier performance, which shall cover the contents of each piece in accordance with 4.2.1
Determined performance levels.
4.1.3 Technical Information
The manufacturer shall provide technical information in accordance with this clause.The technical information shall include.
a) details of the liquid barrier properties of each critical area component;
Note. The information on the liquid barrier performance of each part (determined according to 4.2.1) can be described graphically, or described in text, or both.
Both.
b) details of the liquid barrier performance of each area outside the critical area;
Note. Outside the critical area, the information on the liquid barrier performance of each part (determined according to 4.2.1) can be described graphically, or described in text.
The description can be both.
c) Handling guidelines for reusable medical protective clothing. It should be stated that after the product is processed, it can still maintain its safety and effectiveness.
frequency;
d) Inspection guidelines for reusable medical protective clothing. According to the guidelines, processors can verify that the product maintains its safety and effectiveness;
e) Disposal guidelines for reusable medical protective clothing. When the product is no longer able to demonstrate its stated liquid barrier properties, or
Has reached the end of its indicated use period, the disposer should downgrade it to a non-protective product, rather than reduce the product's liquid protection
Protection performance level.
4.1.4 Training
The manufacturer shall provide technical information and/or training to the end user to explain the barrier performance classification system and its significance. By product
The barrier performance level, and the possible exposure of medical personnel to blood, body fluids or other potentially infectious agents in the corresponding environment of use.
The end user is responsible for selecting the right product.
4.2 Performance requirements
4.2.1 Barrier performance
4.2.1.1 General
The barrier performance levels of protective and non-protective areas of protective clothing shall be determined in accordance with 5.2.1.
The protective clothing should be classified and labeled according to the barrier performance in key areas. Shall include the attachment points for seams and attachments
Liquid barrier performance of all critical area components. The minimum barrier performance of components in key areas of the product should be stated in digital form, etc.
level. It is not necessary to determine the liquid barrier performance level of the joint between the critical area and other protected areas, and the joint between the critical area and the non-protected area
(See 4.2.3). It should be marked according to the performance of the product at the end of the period of use (such as after treatment according to the method and number of times recommended by the manufacturer).
Performance) to determine the level of liquid barrier performance of reusable medical protective clothing.
4.2.1.2 Level of barrier performance
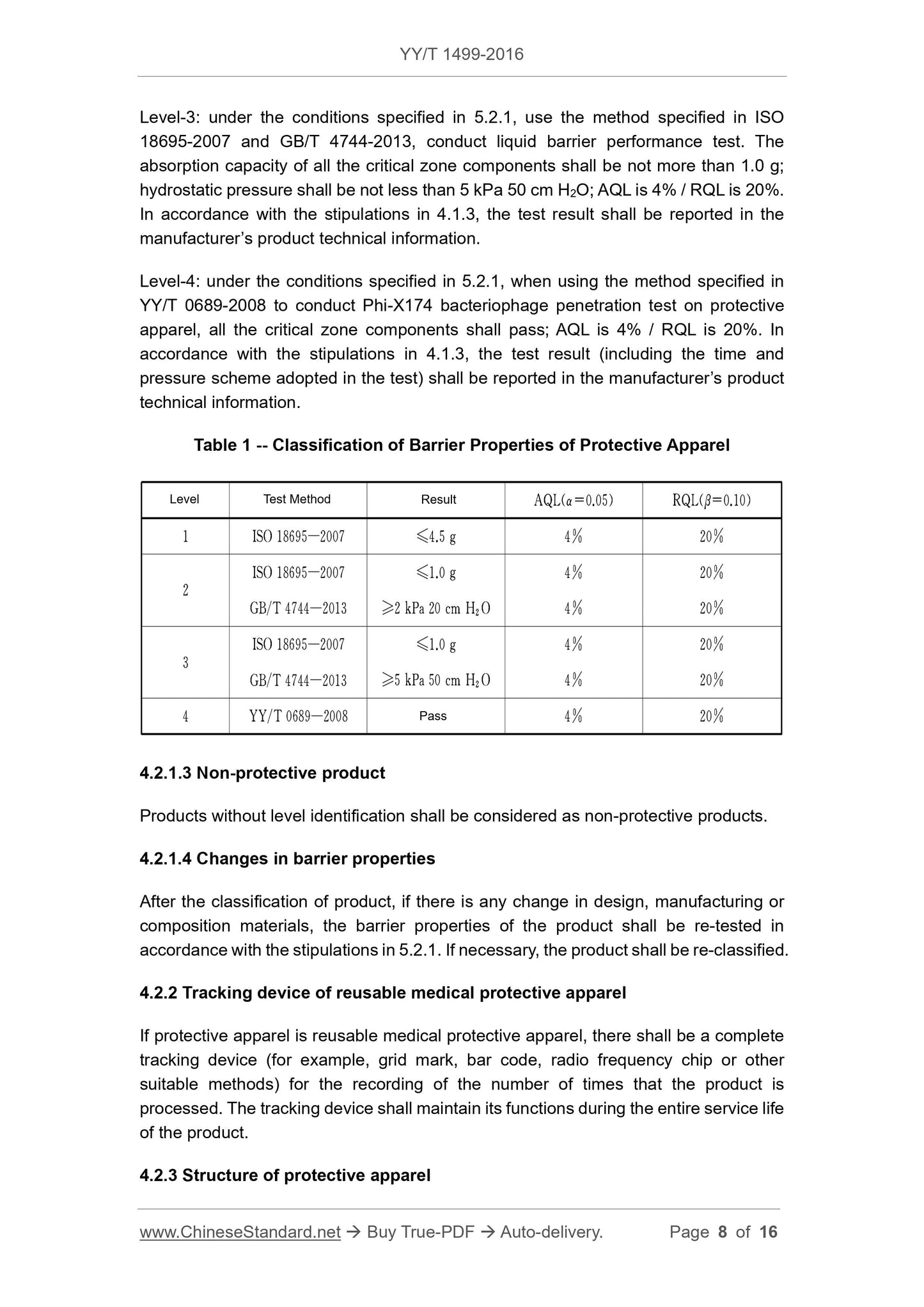
Sampling and testing of critical areas of protective clothing shall be performed in accordance with the provisions of 5.2.1, and classified according to the following provisions and Table 1.
Level 1. Under the conditions specified in 5.2.1, use the method specified in ISO 18695-2007 for liquid barrier performance testing, all key
The adsorption capacity of the zone component should be no more than 4.5g, and the AQL is 4%/RQL is 20%. According to 4.1.3, the test results shall be in the manufacturer's
Reported in product technical data.
Level 2. Under the conditions specified in 5.2.1, use the method specified in ISO 18695-2007 and GB/T 4744-2013 for liquid resistance
In the performance test, the adsorption capacity of all key area components should be no more than 1.0g, and the hydrostatic pressure should be no less than 2kPa20cm H2O. The AQL is
4%/RQL is 20%. According to 4.1.3, the test results shall be reported in the manufacturer's product technical information.
Level 3. Under the conditions specified in 5.2.1, use the method specified in ISO 18695-2007 and GB/T 4744-2013 for liquid resistance
In the performance test, the adsorption capacity of all key area components should be no more than 1.0g, and the hydrostatic pressure should be no less than 5kPa50cm H2O. AQL is
4%/RQL is 20%. According to 4.1.3, the test results shall be reported in the manufacturer's product technical information.
Level 4. Under the conditions specified in 5.2.1, use the method specified in YY/T 0689-2008 to perform phage Phi-X174 on the protective clothing
During penetration testing, all critical area components should pass, with an AQL of 4%/RQL of 20%. According to 4.1.3, test results (including test
The test time and pressure scheme used) shall be reported in the manufacturer's product technical data.
Table 1 Classification of barrier performance of protective clothing
Grade test method result AQL (α = 0.05) RQL (β = 0.10)
1 ISO 18695-2007 ≤4.5g 4% 20%
ISO 18695-2007 ≤1.0g 4% 20%
GB/T 4744-2013 ≥2kPa20cmH2O 4% 20%
ISO 18695-2007 ≤1.0g 4% 20%
GB/T 4744-2013 ≥5kPa50cmH2O 4% 20%
4 YY/T 0689-2008 Pass 4% 20%
4.2.1.3 Non-Protective Products
Products without a rating mark shall be considered non-protective.
4.2.1.4 Changing the barrier performance
After the product is classified, if there are any changes in the design, manufacturing or composition materials, the product should be retested in accordance with 5.2.1
The barrier properties should be reclassified if necessary.
4.2.2 Tracking device for reusable medical protective clothing
Protective clothing for reusable medical protective clothing should have a complete tracking device (e.g. grid marking, barcode, RF chip or
Other suitable methods) to record the number of times the product has been processed. The tracking device shall maintain its function throughout the life of the product.
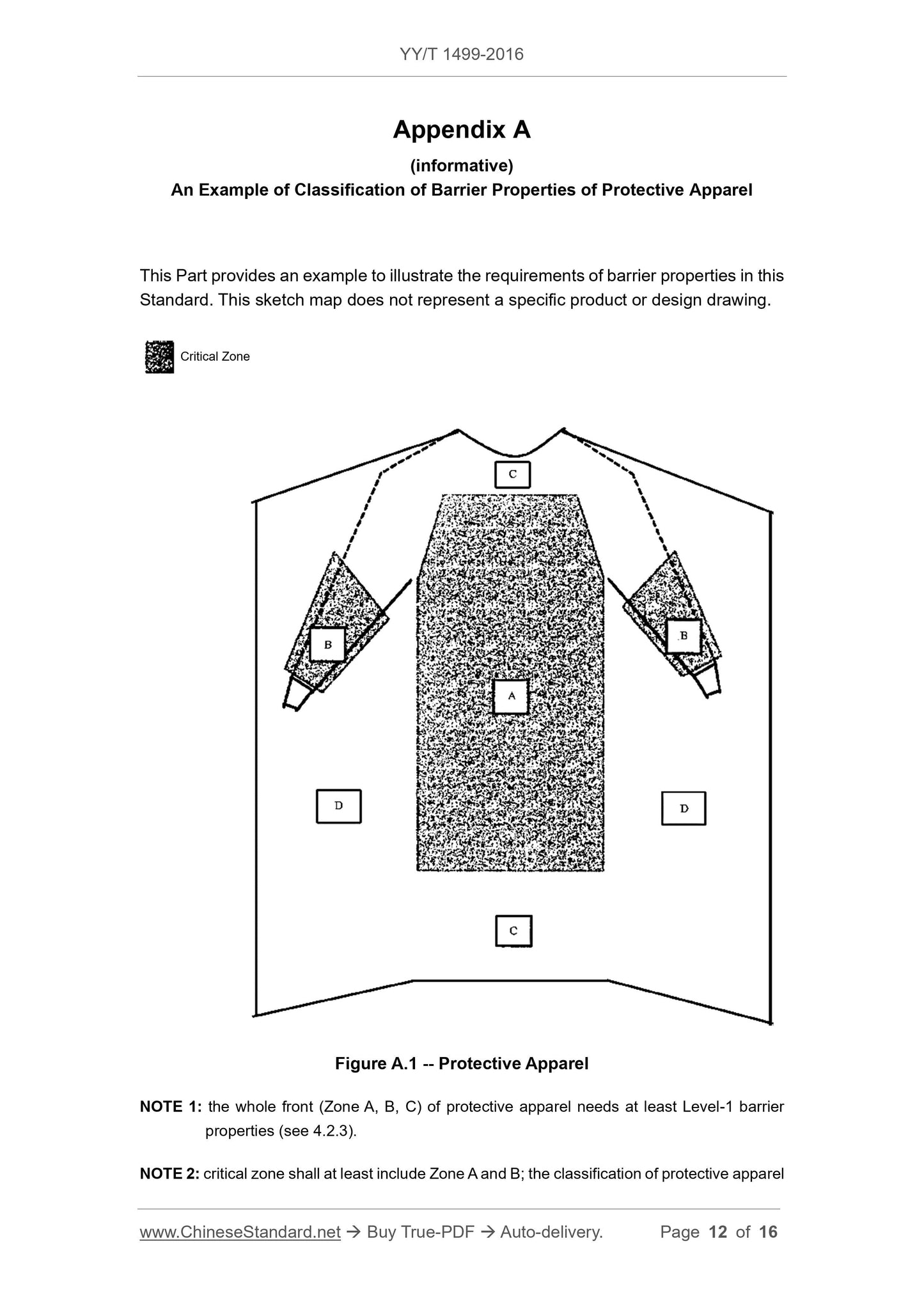
4.2.3 Protective clothing structure
The key areas of protective clothing (excluding isolation clothing) include at least the area of the front from the chest to the knee and the sleeve from the cuff to the elbow.
region. The manufacturer shall specify the exact dimensions of the critical area and shall also provide the barrier properties of each critical area component as specified in 4.1.3 a)
information. The manufacturer shall also provide detailed information on barrier performance in non-critical areas in accordance with 4.1.3b).
An example of the above is given in Appendix A.
5 Test method
5.1 Identification
Visual inspection shall meet the requirements of 4.1.
5.2 Performance
5.2.1 Barrier performance
5.2.1.1 Sampling
Before testing the liquid barrier performance of the product, the following principles should be taken for sampling.
a) Sample size. The manufacturer's determination of the barrier performance levels of components or other areas of the product in key areas meets 4.1 and 4.2.1
When required, an acceptable statistical design and analysis method should be used to select an AQL that is not greater than 4%/RQL is not large
At 20% of the test sample size.
b) Test samples. If the product uses different materials in different parts, then samples should be taken at each part separately, regardless of whether it is in a critical area
Still outside the region.
Samples should be taken on different products of the same lot. If multiple tests must be performed (e.g. the composition of a critical area with more than one component, e.g.
Including materials, joints, joints, etc.), then you can take a sample of each component on the same sample. Each material and group tested
The points shall meet the requirements of the design and structure of the final product. In all barrier performance tests, the outermost surface of the specimen shall be used as the test layer.
If the test area is a reinforcing layer or is composed of multiple layers of material, they should be stacked in the correct order and tested together. When the material or component is pressed
When testing according to ISO 18695-2007 and GB/T 4744-2013, samples should be placed in the same way for each test. press
When testing according to ISO 18695-2007, the joints of the seam and all accessories must be in the middle of the sample, and the sample size should be 17.8cm ×
33.0cm. For testing according to GB/T 4744-2013, the connection point of the seam and accessories must be in the middle of the sample, and the size of the sample should be
20.0cm × 20.0cm.
Note. If it can be proved that the simulated samples can represent real samples, then samples that simulate key design and structural characteristics can also be used.
c) Sampling plan. The samples shall be randomly selected according to the statistical sampling plan, and the sampling plan shall be applicable to the type of data obtained. suitable
See Appendix B for the sampling plan. This standard stipulates that the AQL is 4.0%, the acceptance level is 95% (α = 0.05), and the RQL is 20%.
The acceptance level is 10% (β = 0.10). When initial product classification, a sampling method should be established for each critical area component
The sampling plan should cover multiple batches of samples.
5.2.1.2 Test method for barrier performance of protective clothing
Level 1. critical area components of protective clothing should be tested in accordance with ISO 18695-2007; levels 2 and 3. critical area groups of protective clothing
Tested in accordance with ISO 18695-2007 and GB/T 4744-2013; Level 4. Protective clothing shall pass YY/T 0689-2008
test.
When performing ISO 18695-2007 testing, absorbent paper that meets the following requirements can be used.
--- It should not deform after absorbing water;
--- Water absorption time sho...
Get Quotation: Click YY/T 1499-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1499-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1499-2016: Liquid Barrier Performance and Classification of Protective Apparel Intended for Use in Health Care Facilities
YY/T 1499-2016
Liquid barrier performance and classification of protective apparel intended for use in health care facilities
ICS 11.120
C48
People's Republic of China Pharmaceutical Industry Standard
Liquid barrier properties and classification of medical protective clothing
2016-07-29 release
2017-06-01 Implementation
Published by the State Food and Drug Administration
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some elements of this document may involve patents. The issuer of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the State Food and Drug Administration Beijing Medical Device Quality Supervision and Inspection Center.
This standard was drafted. Beijing Medical Device Inspection Institute.
The main drafters of this standard. Huang Yongfu, Yue Weihua, Jiang Hongxia, Hu Guangyong.
Liquid barrier properties and classification of medical protective clothing
1 Scope
This standard specifies the classification of liquid barrier properties of medical protective clothing and related labeling requirements.
This standard applies to protective clothing marked with liquid barrier properties or liquid bio-microbial barrier properties.
This standard does not apply to other protective equipment used by medical personnel, such as. (1) not labeled or not used for the prevention of liquid or microbial barriers
Protective equipment (such as radiation protective clothing); (2) Appliances or equipment for handling hazardous chemicals, chemotherapy drugs or hazardous waste.
This standard does not apply to medical protective clothing that protects against penetration by solid particles or solid microorganisms.
This standard does not address guidelines for the proper handling or disposal of reusable medical protective clothing by medical institutions.
2 Normative references
The following documents are essential for the application of this document. For dated references, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 4744-2013 Testing and Evaluation of Textile Waterproofing Performance
YY/T 0689-2008 Blood and body fluid protection equipment Protective clothing material Anti-blood-transmitted pathogen penetration performance test Phi-
X174 bacteriophage test method
ISO 18695-2007 Textiles-Determination of Water Penetration (Textiles-Determinationofresist-
(ancetowaterpenetration-Impactpenetrationtest)
3 terms and definitions
The following terms and definitions apply to this document.
3.1
Barrier properties
Protective clothing is able to resist the ability of liquid or phytobiotic microorganisms to penetrate.
3.2
Otherpotentialyinfectiousmaterials
OPIM
In addition to blood or body fluids, it carries blood-borne pathogens or substances related to the transmission of infectious diseases.
3.3
Criticalzone
Areas on protective clothing that are most likely to come in direct contact with blood, body fluids, and other potentially infectious substances (OPIM).
3.4
Criticalzonecomponent
The critical area contains all parts, including materials, seams and attachment points for attachments.
3.5
Reinforcement area
An area in which one or two layers of the same or different materials as the product itself are added to the protective clothing to enhance or improve the performance of the product.
3.6
Seam seam
A site where two or more pieces of material are joined together.
Note. There are many ways to form seams, including traditional needle and needle stitching, as well as bonding, welding, and false stitching.
4 Requirements
4.1 Identification requirements
4.1.1 Single package
Each piece of protective clothing shall have a permanent identification of the level of liquid barrier performance, and the classification of this performance shall be carried out in accordance with 4.2.1.
If the back panel of the protective clothing cannot meet the requirements of level 1 liquid barrier performance, each protective clothing should have "no liquid protection performance on the back"
Permanent warning sign.
Each piece of protective clothing should be labeled as medical protective clothing.
4.1.2 Outer packaging
The outer packaging of each piece of protective clothing shall have a permanent identification of the level of liquid barrier performance, which shall cover the contents of each piece in accordance with 4.2.1
Determined performance levels.
4.1.3 Technical Information
The manufacturer shall provide technical information in accordance with this clause.The technical information shall include.
a) details of the liquid barrier properties of each critical area component;
Note. The information on the liquid barrier performance of each part (determined according to 4.2.1) can be described graphically, or described in text, or both.
Both.
b) details of the liquid barrier performance of each area outside the critical area;
Note. Outside the critical area, the information on the liquid barrier performance of each part (determined according to 4.2.1) can be described graphically, or described in text.
The description can be both.
c) Handling guidelines for reusable medical protective clothing. It should be stated that after the product is processed, it can still maintain its safety and effectiveness.
frequency;
d) Inspection guidelines for reusable medical protective clothing. According to the guidelines, processors can verify that the product maintains its safety and effectiveness;
e) Disposal guidelines for reusable medical protective clothing. When the product is no longer able to demonstrate its stated liquid barrier properties, or
Has reached the end of its indicated use period, the disposer should downgrade it to a non-protective product, rather than reduce the product's liquid protection
Protection performance level.
4.1.4 Training
The manufacturer shall provide technical information and/or training to the end user to explain the barrier performance classification system and its significance. By product
The barrier performance level, and the possible exposure of medical personnel to blood, body fluids or other potentially infectious agents in the corresponding environment of use.
The end user is responsible for selecting the right product.
4.2 Performance requirements
4.2.1 Barrier performance
4.2.1.1 General
The barrier performance levels of protective and non-protective areas of protective clothing shall be determined in accordance with 5.2.1.
The protective clothing should be classified and labeled according to the barrier performance in key areas. Shall include the attachment points for seams and attachments
Liquid barrier performance of all critical area components. The minimum barrier performance of components in key areas of the product should be stated in digital form, etc.
level. It is not necessary to determine the liquid barrier performance level of the joint between the critical area and other protected areas, and the joint between the critical area and the non-protected area
(See 4.2.3). It should be marked according to the performance of the product at the end of the period of use (such as after treatment according to the method and number of times recommended by the manufacturer).
Performance) to determine the level of liquid barrier performance of reusable medical protective clothing.
4.2.1.2 Level of barrier performance
Sampling and testing of critical areas of protective clothing shall be performed in accordance with the provisions of 5.2.1, and classified according to the following provisions and Table 1.
Level 1. Under the conditions specified in 5.2.1, use the method specified in ISO 18695-2007 for liquid barrier performance testing, all key
The adsorption capacity of the zone component should be no more than 4.5g, and the AQL is 4%/RQL is 20%. According to 4.1.3, the test results shall be in the manufacturer's
Reported in product technical data.
Level 2. Under the conditions specified in 5.2.1, use the method specified in ISO 18695-2007 and GB/T 4744-2013 for liquid resistance
In the performance test, the adsorption capacity of all key area components should be no more than 1.0g, and the hydrostatic pressure should be no less than 2kPa20cm H2O. The AQL is
4%/RQL is 20%. According to 4.1.3, the test results shall be reported in the manufacturer's product technical information.
Level 3. Under the conditions specified in 5.2.1, use the method specified in ISO 18695-2007 and GB/T 4744-2013 for liquid resistance
In the performance test, the adsorption capacity of all key area components should be no more than 1.0g, and the hydrostatic pressure should be no less than 5kPa50cm H2O. AQL is
4%/RQL is 20%. According to 4.1.3, the test results shall be reported in the manufacturer's product technical information.
Level 4. Under the conditions specified in 5.2.1, use the method specified in YY/T 0689-2008 to perform phage Phi-X174 on the protective clothing
During penetration testing, all critical area components should pass, with an AQL of 4%/RQL of 20%. According to 4.1.3, test results (including test
The test time and pressure scheme used) shall be reported in the manufacturer's product technical data.
Table 1 Classification of barrier performance of protective clothing
Grade test method result AQL (α = 0.05) RQL (β = 0.10)
1 ISO 18695-2007 ≤4.5g 4% 20%
ISO 18695-2007 ≤1.0g 4% 20%
GB/T 4744-2013 ≥2kPa20cmH2O 4% 20%
ISO 18695-2007 ≤1.0g 4% 20%
GB/T 4744-2013 ≥5kPa50cmH2O 4% 20%
4 YY/T 0689-2008 Pass 4% 20%
4.2.1.3 Non-Protective Products
Products without a rating mark shall be considered non-protective.
4.2.1.4 Changing the barrier performance
After the product is classified, if there are any changes in the design, manufacturing or composition materials, the product should be retested in accordance with 5.2.1
The barrier properties should be reclassified if necessary.
4.2.2 Tracking device for reusable medical protective clothing
Protective clothing for reusable medical protective clothing should have a complete tracking device (e.g. grid marking, barcode, RF chip or
Other suitable methods) to record the number of times the product has been processed. The tracking device shall maintain its function throughout the life of the product.
4.2.3 Protective clothing structure
The key areas of protective clothing (excluding isolation clothing) include at least the area of the front from the chest to the knee and the sleeve from the cuff to the elbow.
region. The manufacturer shall specify the exact dimensions of the critical area and shall also provide the barrier properties of each critical area component as specified in 4.1.3 a)
information. The manufacturer shall also provide detailed information on barrier performance in non-critical areas in accordance with 4.1.3b).
An example of the above is given in Appendix A.
5 Test method
5.1 Identification
Visual inspection shall meet the requirements of 4.1.
5.2 Performance
5.2.1 Barrier performance
5.2.1.1 Sampling
Before testing the liquid barrier performance of the product, the following principles should be taken for sampling.
a) Sample size. The manufacturer's determination of the barrier performance levels of components or other areas of the product in key areas meets 4.1 and 4.2.1
When required, an acceptable statistical design and analysis method should be used to select an AQL that is not greater than 4%/RQL is not large
At 20% of the test sample size.
b) Test samples. If the product uses different materials in different parts, then samples should be taken at each part separately, regardless of whether it is in a critical area
Still outside the region.
Samples should be taken on different products of the same lot. If multiple tests must be performed (e.g. the composition of a critical area with more than one component, e.g.
Including materials, joints, joints, etc.), then you can take a sample of each component on the same sample. Each material and group tested
The points shall meet the requirements of the design and structure of the final product. In all barrier performance tests, the outermost surface of the specimen shall be used as the test layer.
If the test area is a reinforcing layer or is composed of multiple layers of material, they should be stacked in the correct order and tested together. When the material or component is pressed
When testing according to ISO 18695-2007 and GB/T 4744-2013, samples should be placed in the same way for each test. press
When testing according to ISO 18695-2007, the joints of the seam and all accessories must be in the middle of the sample, and the sample size should be 17.8cm ×
33.0cm. For testing according to GB/T 4744-2013, the connection point of the seam and accessories must be in the middle of the sample, and the size of the sample should be
20.0cm × 20.0cm.
Note. If it can be proved that the simulated samples can represent real samples, then samples that simulate key design and structural characteristics can also be used.
c) Sampling plan. The samples shall be randomly selected according to the statistical sampling plan, and the sampling plan shall be applicable to the type of data obtained. suitable
See Appendix B for the sampling plan. This standard stipulates that the AQL is 4.0%, the acceptance level is 95% (α = 0.05), and the RQL is 20%.
The acceptance level is 10% (β = 0.10). When initial product classification, a sampling method should be established for each critical area component
The sampling plan should cover multiple batches of samples.
5.2.1.2 Test method for barrier performance of protective clothing
Level 1. critical area components of protective clothing should be tested in accordance with ISO 18695-2007; levels 2 and 3. critical area groups of protective clothing
Tested in accordance with ISO 18695-2007 and GB/T 4744-2013; Level 4. Protective clothing shall pass YY/T 0689-2008
test.
When performing ISO 18695-2007 testing, absorbent paper that meets the following requirements can be used.
--- It should not deform after absorbing water;
--- Water absorption time sho...
Share