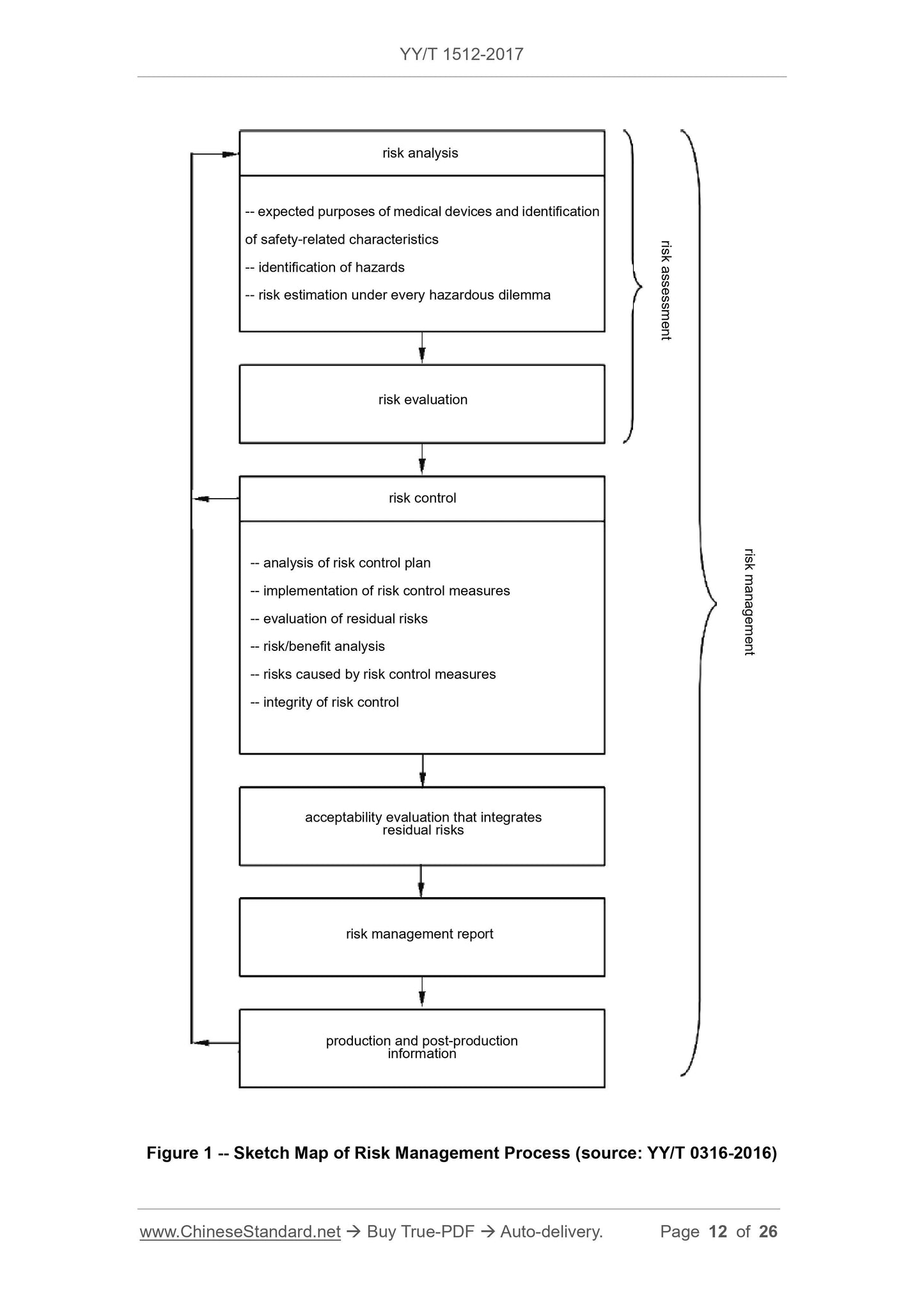
1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1512-2017 English PDF (YYT1512-2017)
YY/T 1512-2017 English PDF (YYT1512-2017)
Regular price
$145.00 USD
Regular price
Sale price
$145.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1512-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1512-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1512-2017: Biological evaluation of medical devices—Guidance on the conduct of biological evaluation within a risk management process
YY/T 1512-2017
Biological evaluation of medical devices-Guidance on the conduct of biological evaluation within a risk management process
ICS 11.040.01
C30
People's Republic of China Pharmaceutical Industry Standard
Medical device biology evaluation risk management
Guide to the implementation of biological evaluation in the process
(ISO /T R15499..2016, MOD)
Released on.2017-07-17
2018-07-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard uses the redrafting method to modify the use of ISO /T R15499.2016 "Medical Device Biological Evaluation Risk Management Process
Implementation Guide for Physical Evaluation.
The technical differences between this standard and ISO /T R15499.2016 are as follows.
---About the normative reference documents, this standard has made technical adjustments to adapt to China's technical conditions, adjustments
The situation is reflected in Chapter 2, “Regulatory Citations”, and the specific adjustments are as follows.
● Replace ISO 10993-1.2009 with GB/T 16886.1-2011 equivalent to the international standard;
● Replace ISO 10993-7 with GB/T 16886.7 equivalent to the international standard;
● Replace ISO 10993-9 with GB/T 16886.9 equivalent to the international standard;
● Replace ISO 10993-12 with GB/T 16886.12 equivalent to the international standard;
● Replace ISO 10993-13 with GB/T 16886.13 equivalent to the international standard;
● Replace ISO 10993-14 with GB/T 16886.14 equivalent to the international standard;
● Replace ISO 10993-15 with GB/T 16886.15 equivalent to the international standard;
● Replace ISO 10993-16 with GB/T 16886.16 equivalent to the international standard;
● Replace ISO 10993-17 with GB/T 16886.17 equivalent to the international standard;
● Replace ISO 10993-18 with GB/T 16886.18 equivalent to the international standard;
● Replace ISO 10993-19 with GB/T 16886.19 equivalent to the international standard;
● Replaced ISO 13485.2016 with YY/T 0287-2017 equivalent to the international standard;
● Replaced ISO 14971.2007 with YY/T 0316-2016 equivalent to the international standard;
● Replaced ISO /IEC 17025 with CNAS-CL01 equivalent to the international standard.
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Medical Device Biology Evaluation Standardization Technical Committee (SAC/TC248).
This standard was drafted. Shandong Medical Device Product Quality Inspection Center.
The main drafters of this standard. Liu Chenghu, Hou Li, Wu Ping.
introduction
0.1 General
This standard provides guidance for the implementation of biological evaluation of medical devices in accordance with the requirements of GB/T 16886.1-2011. although
GB/T 16886.1-2011 provides a general framework for the biological evaluation of medical devices, but it needs to be more detailed when actually using the standard.
A fine guide. Therefore, this standard is developed to provide guidance for users of GB/T 16886.1-2011. This standard can be used to better understand
The requirements of GB/T 16886.1-2011 and the various ways of meeting the requirements of GB/T 16886.1-2011 are described.
Biological evaluation is a set of design verification activities within the broader risk management process. Therefore, this standard includes
The guidelines for GB/T 16886.1-2011 are applied in the risk management process according to the requirements of YY/T 0316-2016. As a medical device
Part of the overall evaluation and development of the machinery, in the process of establishing and maintaining a risk assessment for biological evaluation, consider the description of this standard.
Concepts and methods.
With the development of science, we have mastered the basic mechanism of organizational response, and biological evaluation can be established in related departments.
Review of data, chemical analysis, and required in vitro and in vivo tests. GB/T 16886.1-2011 specifies a plan
The framework for biological evaluation, by prioritizing the use of chemical composition tests and in vitro models using information that is equally relevant to in vivo models.
The number of animals tested was the least and the degree of exposure was the lowest. Choosing the right method for a particular medical device will depend on the characteristics of the device, the phase
Access to scientific data and risk assessment.
Appropriate regulatory requirements and regulatory guidelines should be considered when determining the availability of the guidelines in this standard.
The organization may voluntarily adopt the guidelines of this standard in whole or in part into its risk management process.
The guidelines included in this standard can be used as background information by the assessor of the risk management process, the conformity assessment in the organization, and the regulatory authority.
0.2 Relationship between other standards, guidelines and regulatory requirements
The relationship between GB/T 16886.1-2011, this standard, biological evaluation standards for medical devices and general risk management standards is summarized as follows.
--- This standard provides guidance for the application of GB/T 16886.1-2011;
--- Biological evaluation is an integral part of risk management. This document contains biological evaluation using YY/T 0316-2016.
guide.
Medical device biology evaluation risk management
Guide to the implementation of biological evaluation in the process
1 Scope
This standard applies to the biological evaluation of medical devices in accordance with the requirements of GB/T 16886.1-2011. This standard has not been increased or
Change the requirements of GB/T 16886.1-2011. This standard does not include requirements for regulatory inspection or certification assessment activities.
This standard applies to all biology of all types of medical devices, including active, passive, implanted and non-implanted medical devices.
Evaluation.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 16886.1-2011 Biological evaluation of medical devices - Part 1 Evaluation and testing in the process of risk management
(ISO 10993-1.2009, IDT)
GB/T 16886.7 Biological evaluation of medical devices - Part 7. Resin residues from ethylene oxide (GB/T 16886.7-2015,
ISO 10993-7.2008, IDT)
GB/T 16886.9 Biological evaluation of medical devices - Part 9. Qualitative and quantitative framework for potential degradation products
(GB/T 16886.9-2001, ISO 10993-9..1999, IDT)
GB/T 16886.12 Biological evaluation of medical devices - Part 12. Sample preparation and reference samples (GB/T 16886.12-
2005, ISO 10993-12.2002, IDT)
GB/T 16886.13 Biological evaluation of medical devices - Part 13. Qualification and quantification of degradation products of polymer medical devices
(GB/T 16886.13-2001, ISO 10993-13.1998, IDT)
GB/T 16886.14 Biological evaluation of medical devices - Part 14. Qualification and quantification of ceramic degradation products
(GB/T 16886.14-2003, ISO 10993-14..2001, IDT)
GB/T 16886.15 Biological evaluation of medical devices - Part 15. Qualification and quantification of degradation products of metals and alloys
(GB/T 16886.15-2003, ISO 10993-15..2000, IDT)
GB/T 16886.16 Biological evaluation of medical devices - Part 16. Design and analysis of toxic kinetics of degradation products and solubles
(GB/T 16886.16-2003, ISO 10993-16..1997, IDT)
GB/T 16886.17 Biological evaluation of medical devices - Part 17. Establishment of leaching allowances
(GB/T 16886.17-2005, ISO 10993-17.2002, IDT)
GB/T 16886.18 Biological evaluation of medical devices - Part 18. Chemical characterization of materials (GB/T 16886.18-2011,
ISO 10993-18.2006, IDT)
GB/T 16886.19 Biological evaluation of medical devices - Part 19. Physical chemistry, morphological and surface characterization of materials
(GB/T 16886.19-2011, ISO /T S10993-19.2006, IDT)
YY/T 0287-2017 Medical device quality management system for regulatory requirements (ISO 13485.2016, IDT)
YY/T 0316-2016 Medical Device Risk Management for Medical Devices (ISO 14971.2007, IDT)
CNAS-CL01 Laboratory Accreditation Guidelines for Testing and Calibration (CNAS-CL01.2006, ISO /IEC 17025.2005,
IDT)
3 Terms and definitions
The following terms and definitions as defined in GB/T 16886.1-2011 apply to this document.
3.1
Biocompatibility
The ability of a medical device or material to have a suitable host response in a particular application.
3.2
Biological risk biologicalrisk
The result of a medical device or material interaction that results in a health hazard.
3.3
Biosafety biologicalsafety
There are no unacceptable biological risks.
3.4
Risk assessment riskassessment
Including the entire process of risk analysis and risk assessment.
[ISO /IEC Guide 51-2003, definition 3.11]
3.5
Risk assessment riskvaluation
The process of comparing the estimated risk to a given risk criterion to determine the acceptability of the risk.
[YY/T 0316-2016, definition 2.21]
3.6
Risk management riskmanagement
Systematic use of management policies, procedures, and practices for risk analysis, evaluation, control, and monitoring.
[YY/T 0316-2016, definition 2.22]
3.7
Toxicological hazard toxicologicalhazard
The potential of a compound or material to cause an adverse biological reaction, taking into account the nature of the reaction and the dose required to induce the reaction.
3.8
Toxicological risk toxicologicalrisk
The probability of a certain degree of adverse reaction occurring for a particular level of exposure.
3.9
Risk analysis riskanalysis
The system uses the information available to identify hazards and estimate their risks.
[YY/T 0316-2016, definition 2.17, modified]
4 Biological evaluation is a risk management activity
4.1 General
B.2.2.2 of GB/T 16886.1-2011 describes a manufacturer's ability to identify biological hazards associated with medical devices, estimates
And the ongoing process of assessing their risks, controlling these risks, and monitoring the effectiveness of controls. By weighing the risks and benefits of medical devices
Protecting patients is an essential element of this biological evaluation program. Patients benefit from the use of medical devices while also being exposed to potential
risk. These risks will vary depending on the characteristics and intended use of the particular medical device. The acceptable level of risk for a particular device
The expected benefits of the device are determined.
The consideration of the biological (toxicology) risk of medical devices is only one aspect of risk assessment that should be considered in many aspects. Some situations
In particular, it is necessary to consider the benefits of other properties besides the biosafety of materials. For example, the best biosafety material possible
Does not have acceptable mechanical strength. In this case, it is necessary to consider replacing a more robust biosafety acceptable material. real
The premise of biological evaluation is to recognize that this is only part of the overall risk management process required for the design and development of medical devices.
Material selection and risk analysis are an integral part of the medical device design process. The choice of materials plays a role in biosafety evaluation
Decisive role. And when you start in a systematic way, you can collect relevant data. According to YY/T 0287-2017 and
Y/T 0316-2016, criteria for accepting biological (toxicology) risks should be determined at the beginning of the design process. Due to initial materials, formulations and
Variations in the process may affect the biocompatibility of the final product and these considerations should be incorporated into the risk assessment. Design and carry out
Safety assessment to verify that specific safety guidelines are met. As an integral part of the risk management plan, this evaluation revolves around all
Identification of hazards and estimates of associated risks. Adequate risk assessment requires characterization of toxicological hazards and exposure levels.
A major component of hazard identification is material characterization. include.
--- Define and characterize each material (including suitable alternative materials);
--- Identify hazards, additives, processing aids, etc. in materials;
--- Identify the potential of chemicals in the final product (such as chemical reactions between material components or sterilization of the final product)
influences;
--- Identify chemicals that may be released during product use (such as intermediate or final degradation products of a degradable implant);
--- Estimated exposure (total or clinical intake);
--- Review toxicology and other biosafety data (published or available).
Biosafety information to be reviewed includes.
--- toxicological data on related constituent materials or compounds;
--- Previous information on the use of the constituent materials or compounds;
--- Biosafety test data.
It is advisable to conduct a risk assessment of the identified hazards. At this stage, it can be determined whether the material has an inappropriate toxicological risk.
If it can be inferred from the available data that the risk is acceptable, it is not necessary to conduct a toxicological test. If the data is insufficient, then
More information should be obtained. The purpose of the trial is to obtain more data that will help to draw conclusions. Therefore, the principle of the test should be based on
Based on the analysis of relevant risks from existing data.
The results of any test should be assessed. The test report should include a description of the evidence, an assessment of the results, and a qualitative analysis of their acceptability.
assessment.
The assessor should determine if the informa...
Get Quotation: Click YY/T 1512-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1512-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1512-2017: Biological evaluation of medical devices—Guidance on the conduct of biological evaluation within a risk management process
YY/T 1512-2017
Biological evaluation of medical devices-Guidance on the conduct of biological evaluation within a risk management process
ICS 11.040.01
C30
People's Republic of China Pharmaceutical Industry Standard
Medical device biology evaluation risk management
Guide to the implementation of biological evaluation in the process
(ISO /T R15499..2016, MOD)
Released on.2017-07-17
2018-07-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard uses the redrafting method to modify the use of ISO /T R15499.2016 "Medical Device Biological Evaluation Risk Management Process
Implementation Guide for Physical Evaluation.
The technical differences between this standard and ISO /T R15499.2016 are as follows.
---About the normative reference documents, this standard has made technical adjustments to adapt to China's technical conditions, adjustments
The situation is reflected in Chapter 2, “Regulatory Citations”, and the specific adjustments are as follows.
● Replace ISO 10993-1.2009 with GB/T 16886.1-2011 equivalent to the international standard;
● Replace ISO 10993-7 with GB/T 16886.7 equivalent to the international standard;
● Replace ISO 10993-9 with GB/T 16886.9 equivalent to the international standard;
● Replace ISO 10993-12 with GB/T 16886.12 equivalent to the international standard;
● Replace ISO 10993-13 with GB/T 16886.13 equivalent to the international standard;
● Replace ISO 10993-14 with GB/T 16886.14 equivalent to the international standard;
● Replace ISO 10993-15 with GB/T 16886.15 equivalent to the international standard;
● Replace ISO 10993-16 with GB/T 16886.16 equivalent to the international standard;
● Replace ISO 10993-17 with GB/T 16886.17 equivalent to the international standard;
● Replace ISO 10993-18 with GB/T 16886.18 equivalent to the international standard;
● Replace ISO 10993-19 with GB/T 16886.19 equivalent to the international standard;
● Replaced ISO 13485.2016 with YY/T 0287-2017 equivalent to the international standard;
● Replaced ISO 14971.2007 with YY/T 0316-2016 equivalent to the international standard;
● Replaced ISO /IEC 17025 with CNAS-CL01 equivalent to the international standard.
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Medical Device Biology Evaluation Standardization Technical Committee (SAC/TC248).
This standard was drafted. Shandong Medical Device Product Quality Inspection Center.
The main drafters of this standard. Liu Chenghu, Hou Li, Wu Ping.
introduction
0.1 General
This standard provides guidance for the implementation of biological evaluation of medical devices in accordance with the requirements of GB/T 16886.1-2011. although
GB/T 16886.1-2011 provides a general framework for the biological evaluation of medical devices, but it needs to be more detailed when actually using the standard.
A fine guide. Therefore, this standard is developed to provide guidance for users of GB/T 16886.1-2011. This standard can be used to better understand
The requirements of GB/T 16886.1-2011 and the various ways of meeting the requirements of GB/T 16886.1-2011 are described.
Biological evaluation is a set of design verification activities within the broader risk management process. Therefore, this standard includes
The guidelines for GB/T 16886.1-2011 are applied in the risk management process according to the requirements of YY/T 0316-2016. As a medical device
Part of the overall evaluation and development of the machinery, in the process of establishing and maintaining a risk assessment for biological evaluation, consider the description of this standard.
Concepts and methods.
With the development of science, we have mastered the basic mechanism of organizational response, and biological evaluation can be established in related departments.
Review of data, chemical analysis, and required in vitro and in vivo tests. GB/T 16886.1-2011 specifies a plan
The framework for biological evaluation, by prioritizing the use of chemical composition tests and in vitro models using information that is equally relevant to in vivo models.
The number of animals tested was the least and the degree of exposure was the lowest. Choosing the right method for a particular medical device will depend on the characteristics of the device, the phase
Access to scientific data and risk assessment.
Appropriate regulatory requirements and regulatory guidelines should be considered when determining the availability of the guidelines in this standard.
The organization may voluntarily adopt the guidelines of this standard in whole or in part into its risk management process.
The guidelines included in this standard can be used as background information by the assessor of the risk management process, the conformity assessment in the organization, and the regulatory authority.
0.2 Relationship between other standards, guidelines and regulatory requirements
The relationship between GB/T 16886.1-2011, this standard, biological evaluation standards for medical devices and general risk management standards is summarized as follows.
--- This standard provides guidance for the application of GB/T 16886.1-2011;
--- Biological evaluation is an integral part of risk management. This document contains biological evaluation using YY/T 0316-2016.
guide.
Medical device biology evaluation risk management
Guide to the implementation of biological evaluation in the process
1 Scope
This standard applies to the biological evaluation of medical devices in accordance with the requirements of GB/T 16886.1-2011. This standard has not been increased or
Change the requirements of GB/T 16886.1-2011. This standard does not include requirements for regulatory inspection or certification assessment activities.
This standard applies to all biology of all types of medical devices, including active, passive, implanted and non-implanted medical devices.
Evaluation.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 16886.1-2011 Biological evaluation of medical devices - Part 1 Evaluation and testing in the process of risk management
(ISO 10993-1.2009, IDT)
GB/T 16886.7 Biological evaluation of medical devices - Part 7. Resin residues from ethylene oxide (GB/T 16886.7-2015,
ISO 10993-7.2008, IDT)
GB/T 16886.9 Biological evaluation of medical devices - Part 9. Qualitative and quantitative framework for potential degradation products
(GB/T 16886.9-2001, ISO 10993-9..1999, IDT)
GB/T 16886.12 Biological evaluation of medical devices - Part 12. Sample preparation and reference samples (GB/T 16886.12-
2005, ISO 10993-12.2002, IDT)
GB/T 16886.13 Biological evaluation of medical devices - Part 13. Qualification and quantification of degradation products of polymer medical devices
(GB/T 16886.13-2001, ISO 10993-13.1998, IDT)
GB/T 16886.14 Biological evaluation of medical devices - Part 14. Qualification and quantification of ceramic degradation products
(GB/T 16886.14-2003, ISO 10993-14..2001, IDT)
GB/T 16886.15 Biological evaluation of medical devices - Part 15. Qualification and quantification of degradation products of metals and alloys
(GB/T 16886.15-2003, ISO 10993-15..2000, IDT)
GB/T 16886.16 Biological evaluation of medical devices - Part 16. Design and analysis of toxic kinetics of degradation products and solubles
(GB/T 16886.16-2003, ISO 10993-16..1997, IDT)
GB/T 16886.17 Biological evaluation of medical devices - Part 17. Establishment of leaching allowances
(GB/T 16886.17-2005, ISO 10993-17.2002, IDT)
GB/T 16886.18 Biological evaluation of medical devices - Part 18. Chemical characterization of materials (GB/T 16886.18-2011,
ISO 10993-18.2006, IDT)
GB/T 16886.19 Biological evaluation of medical devices - Part 19. Physical chemistry, morphological and surface characterization of materials
(GB/T 16886.19-2011, ISO /T S10993-19.2006, IDT)
YY/T 0287-2017 Medical device quality management system for regulatory requirements (ISO 13485.2016, IDT)
YY/T 0316-2016 Medical Device Risk Management for Medical Devices (ISO 14971.2007, IDT)
CNAS-CL01 Laboratory Accreditation Guidelines for Testing and Calibration (CNAS-CL01.2006, ISO /IEC 17025.2005,
IDT)
3 Terms and definitions
The following terms and definitions as defined in GB/T 16886.1-2011 apply to this document.
3.1
Biocompatibility
The ability of a medical device or material to have a suitable host response in a particular application.
3.2
Biological risk biologicalrisk
The result of a medical device or material interaction that results in a health hazard.
3.3
Biosafety biologicalsafety
There are no unacceptable biological risks.
3.4
Risk assessment riskassessment
Including the entire process of risk analysis and risk assessment.
[ISO /IEC Guide 51-2003, definition 3.11]
3.5
Risk assessment riskvaluation
The process of comparing the estimated risk to a given risk criterion to determine the acceptability of the risk.
[YY/T 0316-2016, definition 2.21]
3.6
Risk management riskmanagement
Systematic use of management policies, procedures, and practices for risk analysis, evaluation, control, and monitoring.
[YY/T 0316-2016, definition 2.22]
3.7
Toxicological hazard toxicologicalhazard
The potential of a compound or material to cause an adverse biological reaction, taking into account the nature of the reaction and the dose required to induce the reaction.
3.8
Toxicological risk toxicologicalrisk
The probability of a certain degree of adverse reaction occurring for a particular level of exposure.
3.9
Risk analysis riskanalysis
The system uses the information available to identify hazards and estimate their risks.
[YY/T 0316-2016, definition 2.17, modified]
4 Biological evaluation is a risk management activity
4.1 General
B.2.2.2 of GB/T 16886.1-2011 describes a manufacturer's ability to identify biological hazards associated with medical devices, estimates
And the ongoing process of assessing their risks, controlling these risks, and monitoring the effectiveness of controls. By weighing the risks and benefits of medical devices
Protecting patients is an essential element of this biological evaluation program. Patients benefit from the use of medical devices while also being exposed to potential
risk. These risks will vary depending on the characteristics and intended use of the particular medical device. The acceptable level of risk for a particular device
The expected benefits of the device are determined.
The consideration of the biological (toxicology) risk of medical devices is only one aspect of risk assessment that should be considered in many aspects. Some situations
In particular, it is necessary to consider the benefits of other properties besides the biosafety of materials. For example, the best biosafety material possible
Does not have acceptable mechanical strength. In this case, it is necessary to consider replacing a more robust biosafety acceptable material. real
The premise of biological evaluation is to recognize that this is only part of the overall risk management process required for the design and development of medical devices.
Material selection and risk analysis are an integral part of the medical device design process. The choice of materials plays a role in biosafety evaluation
Decisive role. And when you start in a systematic way, you can collect relevant data. According to YY/T 0287-2017 and
Y/T 0316-2016, criteria for accepting biological (toxicology) risks should be determined at the beginning of the design process. Due to initial materials, formulations and
Variations in the process may affect the biocompatibility of the final product and these considerations should be incorporated into the risk assessment. Design and carry out
Safety assessment to verify that specific safety guidelines are met. As an integral part of the risk management plan, this evaluation revolves around all
Identification of hazards and estimates of associated risks. Adequate risk assessment requires characterization of toxicological hazards and exposure levels.
A major component of hazard identification is material characterization. include.
--- Define and characterize each material (including suitable alternative materials);
--- Identify hazards, additives, processing aids, etc. in materials;
--- Identify the potential of chemicals in the final product (such as chemical reactions between material components or sterilization of the final product)
influences;
--- Identify chemicals that may be released during product use (such as intermediate or final degradation products of a degradable implant);
--- Estimated exposure (total or clinical intake);
--- Review toxicology and other biosafety data (published or available).
Biosafety information to be reviewed includes.
--- toxicological data on related constituent materials or compounds;
--- Previous information on the use of the constituent materials or compounds;
--- Biosafety test data.
It is advisable to conduct a risk assessment of the identified hazards. At this stage, it can be determined whether the material has an inappropriate toxicological risk.
If it can be inferred from the available data that the risk is acceptable, it is not necessary to conduct a toxicological test. If the data is insufficient, then
More information should be obtained. The purpose of the trial is to obtain more data that will help to draw conclusions. Therefore, the principle of the test should be based on
Based on the analysis of relevant risks from existing data.
The results of any test should be assessed. The test report should include a description of the evidence, an assessment of the results, and a qualitative analysis of their acceptability.
assessment.
The assessor should determine if the informa...
Share