1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1566.1-2017 English PDF (YYT1566.1-2017)
YY/T 1566.1-2017 English PDF (YYT1566.1-2017)
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1566.1-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1566.1-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1566.1-2017: Autologous blood processing devices for single use - Part 1: Blood cell recovery sets, centrifuge bowl type
YY/T 1566.1-2017
Autologous blood processing devices for single use--Part 1. Blood cell recoery sets, centrifuge bowl type
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Disposable autologous blood processing equipment
Part 1. Centrifugal Cup Blood Cell Harvester
Part 1. Bloodcelrecoerysets,centrifugebowltype
Published on.2017-07-17
2018-07-01 Implementation
The State Food and Drug Administration issued
Directory
Preface III
Introduction IV
1 Range 1
2 Normative references 1
3 Terms and Definitions 1
4 Structure 3
5 Physical requirements 3
5.1 Appearance 3
5.2 Particle Pollution 3
5.2.1 Separation Cup 3
5.2.2 Piping System 3
5.2.3 RBC collection bag 3
5.3 Sealing 3
5.4 Connection strength 4
5.5 Frictional heat 4
5.6 Noise 4
5.7 Residual amount 4
5.8 color standard 4
5.9 Puncher 4
5.10 Dripping Bucket 4
5.11 Flow Regulator 4
5.12 Pump tube elasticity 4
5.13 limit card (if any) 4
5.14 Fixture 5
5.15 Interface 5
5.16 protective cover 5
5.17 RBC collection bag 5
6 Chemical Requirements 5
6.1 Separation Cup and Piping System 5
6.1.1 Reducing substance 5
6.1.2 Metal Ions 5
6.1.3 pH 5
6.1.4 Evaporation residue 5
6.1.5 UV absorbance 5
6.2 RBC collection bag 5
6.3 Ethylene Oxide Residue 5
7 Biological Requirements 6
7.1 Biocompatibility 6
7.2 Aseptic 6
7.3 Bacterial endotoxin 6
8 type inspection 6
9 Signs 6
9.1 General Clause 6
9.2 Single Pack Logo 6
9.3 Shelf Packing Mark 6
10 Packing 7
Appendix A (Informative) Note 8
Appendix B (Normative Appendix) Physical Test 10
Appendix C (Normative) Chemical Test 12
Reference 14
Figure 1 Schematic diagram of example 2
Foreword
YY/T 1566 "Disposable Autologous Blood Processing Apparatus" consists of the following components.
--- Part 1. Centrifugal cup blood cell harvester;
--- Part 2. Blood collection and filtration device (blood reservoir);
This section is Part 1 of YY/T 1566.
This section was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This part is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
This section mainly drafted by. Beijing Jingjing Medical Equipment Co., Ltd., Beijing Wandong Kangyuan Technology Development Co., Ltd., Shandong Province Medical
Device Product Quality Inspection Center.
The main drafters of this section. Zhang Mingli, Zhang Qing, Liu Ye, Yan Chunliang, Sun Lingyan.
introduction
As a disposable autologous blood processing device, a centrifugal cup blood cell recovery device is required to be used together with an autologous blood recovery device. Used outside
When a surgical or traumatic bleeding occurs, the patient's blood is recovered, blood components are separated, washed, replaced, etc., and finally collected into the red blood cells.
Collect the bag for return to the patient. ANSI/AAMIAT6.2013 Clinical Application of Autologous Blood Transfer Device for Autologous Blood Refusion
Please refer to Appendix A for information.
Disposable autologous blood processing equipment
Part 1. Centrifugal Cup Blood Cell Harvester
1 Scope
This part of YY/T 1566 specifies the requirements for a centrifuged cup-type blood cell recovery device used in surgery. It is a one-time use product.
Products to ensure the safety of use with autologous blood recovery machines.
This section applies to centrifuge cups that are intended to be used in conjunction with other autologous blood processing devices and are intended for use with autologous blood recovery equipment.
Blood cell recovery product mainly consists of a pipeline system, a separation cup, a red blood cell collection bag and a waste liquid bag, excluding the blood collection and filtration device
(Blood reservoir).
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 6682 Analysis Laboratory Water Specifications and Test Methods
GB 8368 Single-use infusion set gravity infusion
GB 14232.1-2004 Human blood and blood components Bag-type plastic containers Part 1. Traditional blood bags
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 14233.2 Medical infusion, blood transfusion, injection equipment inspection methods Part 2. Biological test methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB/T 19633.1 Packaging for terminally sterilized medical devices Part 1. Requirements for materials, sterile barrier systems, and packaging systems
YY/T 0466.1 Medical Devices Symbols for Labeling, Marking, and Providing Information on Medical Devices Part 1. General Requirements
YY 0584-2005 Centrifugal Cup Blood Composition Separator
YY/T 0615.1 Requirements for Labeling “Aseptic” Medical Devices Part 1. Requirements for Final Sterilization Medical Devices
ISO 7000 Device Graphic Symbols Registered Symbols (Graphicalsymbolsforuseonequipment-Indexand
Synopsis)
3 Terms and Definitions
The following terms and definitions apply to this document.
3.1
Autologous blood autologousblood
Collect and process the blood of a certain patient and return it to the patient's blood.
3.2
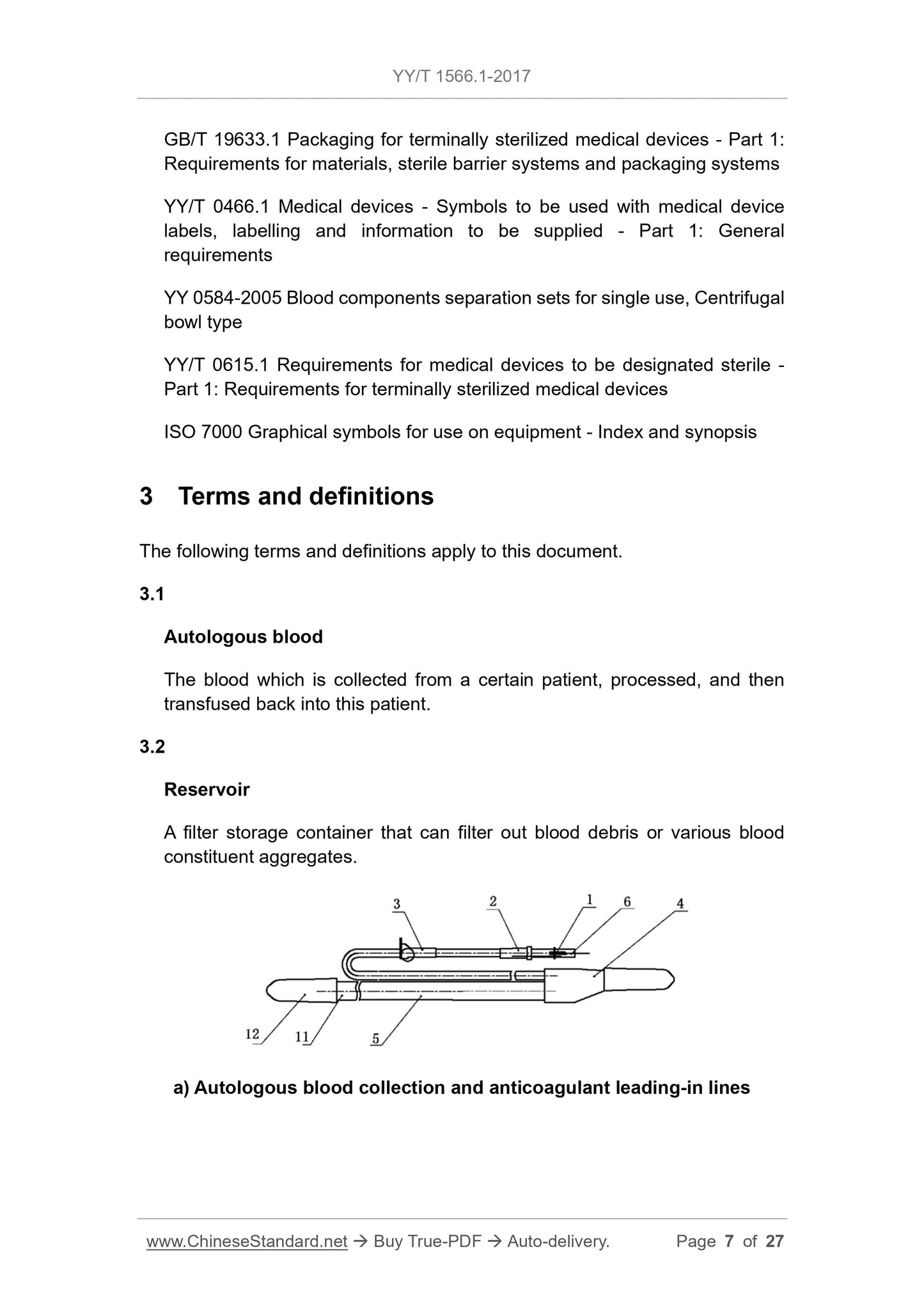
Blood reservoir reservoir
A filter storage container that can filter out blood debris or various blood constituent aggregates.
a) Autologous blood collection and anticoagulant introduction lines
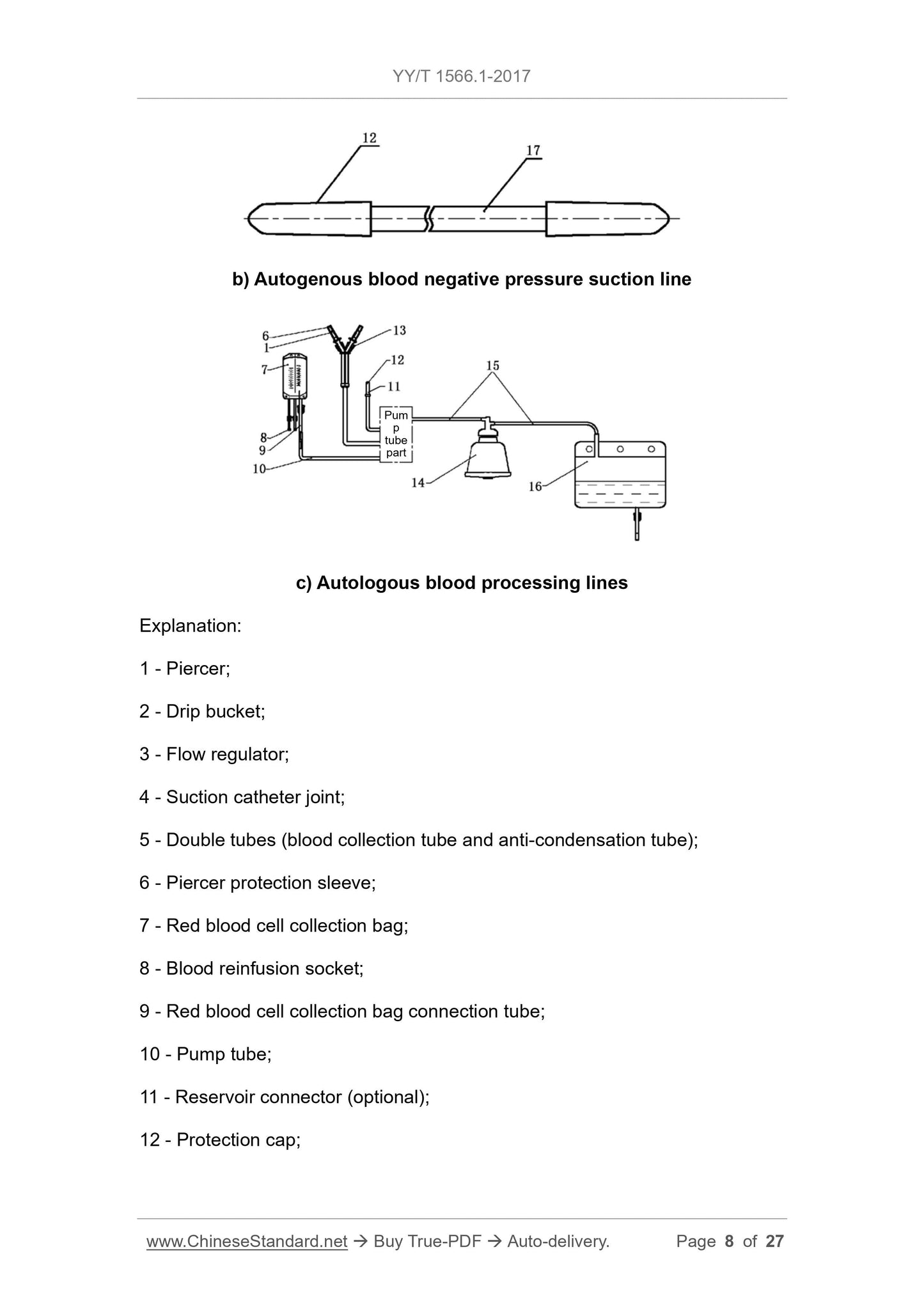
b) Autogenous vacuum suction line
c) Autologous blood processing lines
Explanation.
1 ---Thrors;
2 --- drop bucket;
3 --- flow regulators;
4 --- suction pipe joints;
5 --- double tubes (blood collection tube and anti-condensation tube);
6 --- puncture protector;
7 --- Red blood cell collection bag;
8 --- Blood transfusion socket;
9 --- RBC collection bag connecting pipe;
10 --- pump tube;
11--- Blood reservoir connector (optional);
12---Protection cap;
13---pipe clamps;
14 --- separation cup;
15 --- separation cup connecting pipe;
16--- waste bag;
17--- suction tube.
Figure 1 Schematic diagram
4 Structure
A typical centrifugal cup blood cell recovery device consists of a tubing system (including a separate cup connecting tube, a double tube, a red blood cell collection bag connecting tube, a pump tube,
Auto blood pressure suction tube (optional), separation cup, red cell collection bag, and waste bag. Figure 1 shows a common structure
Intent example.
5 Physical requirements
5.1 Appearance
5.1.1 The cup body and piping system of the separation cup should be transparent or sufficiently transparent to detect water and normal or corrected vision when bubbles pass through.
Air interface.
5.1.2 The surface of the cup body of the separation cup should be smooth and clean. Visual inspection and feel should not cause obvious defects such as spots, impurities, scratches, and burrs.
5.1.3 The piping system shall be plasticized, unobstructed, and free of kinks.
5.1.4 The red blood cell collection bag and the waste bag bag body should have no obvious impurities, spots, bubbles, the inner and outer surfaces should be flat, the inner wall should not adhere, and the heat sealing line should be
Transparent, uniform, hanging holes should be regular. The outer surface of the logo should be clear and easy to identify.
5.1.5 The interface of the separation cup, clamps, and other components should be smooth and free from burrs.
5.2 Particle Pollution
5.2.1 separation cup
When measured in accordance with B.1, the number of particles in 15 μm to 25 μm in the.200 mL eluent should not exceed 6.00 particles/mL, which is greater than 25 μm.
The particles should not exceed 3.00 particles/mL.
5.2.2 Piping System
When measured in accordance with B.2, the number of particles 15 μm to 25 μm on the surface area per square centimeter should not exceed 1.00, greater than 25 μm.
The number of particles should not exceed 0.50.
5.2.3 RBC collection bag
The production of red blood cell collection bags should avoid particulate contamination.
When tested in accordance with the provisions of B.3, red blood cell collection bag should have no visible particles.
Note. Work is under way to establish limits on the number and size of particles. At present, the limits and test methods given in pharmacopoeias can be used (such as the European Pharmacopoeia
The limits and methods of the prescribed formulation).
5.3 Sealing
5.3.1 Separation cup The separation cup shall be free from leakage during the particle contamination test of the separation cup according to B.1.
Note. The sealability of the separation cup and the particulate contamination test are combined.
5.3.2 Close the other ends of the pipelines in the pipeline system, leaving only one end to pass 50kPa air pressure for 15s, underwater inspection should be no
Continuous bubble leaks.
5.3.3 Fill the RBC collection bag with GB/T 6682 water to nominal capacity and seal it. Then put the red blood cell collection bag
Extruded between the two plates, under the conditions of 23 °C ± 5 °C, so that the internal pressure rises above atmospheric pressure 50kPa, for 10min, should be
No leakage.
5.4 Connection strength
5.4.1 The joints (excluding the protective sleeve) of the centrifugal cup blood cell recovery device shall be able to withstand the static axial tension of 15N for 15s.
No breakage and shedding.
5.4.2 Holes or other suspension devices on the red blood cell collection bag should be able to withstand a static axial tension of 10N for 60 minutes without breaking.
5.4.3 Fill the waste bag with water to the maximum mark line, and suspend it for 2 hours under normal use conditions. The suspension device of the waste bag should be continuously
Cracked, and the bag body should have no leakage.
5.5 Frictional heat
When the separation cup is tested according to B.4, the water temperature in the cup should not exceed 37°C.
5.6 Noise
When the separation cup is operated at 5600r/min on the used autologous blood recovery machine, there should be no obvious swing, and the separation cups are at the front, rear, left and right sides.
When the center noise is measured with a sound level meter (A weighting) at a distance of 1m, the maximum noise of the separation cup should not be exceeded when the maximum noise of the main engine does not exceed 60dB.
More than 70dB.
Note. When the manufacturer gives the centrifuge speed, the test is carried out according to the centrifugal speed given by the manufacturer, but not given according to the centrifugal speed of 5600 r/min.
Test.
5.7 Residual amount
When the separation cup is tested according to B.5 or other equivalent methods, the residual amount in the cup should not exceed 22 mL.
5.8 color standard
Each section of the separation cup connecting line (not including the part connected to the waste bag) should adopt different colors of color to provide users with
For identification.
5.9 Puncher
5.9.1 After inserting a blood bag socket conforming to GB 14232.1-2004, the puncture device shall be able to withstand a static tension of 15N for 15 seconds.
5.9.2 When tested in accordance with 5.3 of GB 14232.1-2004, the connection between the puncture device and the blood bag socket shall be free from leakage.
5.10 Dripping Bucket
Droplets should be able to introduce anticoagulant liquids or other liquids into the piping system by means of elastic forces.
5.11 Flow Regulator
Flow regulators should comply with the provisions of GB 8368.
5.12 Pump Tube 1) Elasticity
Pipeline pump tube part should have good elasticity, when the water temperature is 23 °C ± 2 °C, according to A.6 test, the flow rate decreases after 1h
The rate should be less than 5%.
5.13 limit card 2) (if any)
5.13.1 Limit cards should have color identification. The limit card should be adapted to the upper limit device of the autogenous blood recovery machine.
1) Pump tubing refers to the hose installed in the peristaltic pump of the host.
2) The limit card is a plastic part that is installed on the pump tube to limit the displacement of the pump tube and is an optional accessory.
5.13.2 The limit card should be firmly bonded to the pump tube and it is not easy to fall off and shift manually.
5.14 Fixture
When the fixture is closed, it should be able to block the flow of 50kPa gas and liquid, and after opening, it will not damage the pipeline, to ensure the liquid flow.
5.15 Interface
If there is an interface on the piping system that is intended to be connected to the blood reservoir piping, it shall be provided with its associated or manufacturer-specified blood reservoir interface.
Match, according to the instructions for use, should be subjected to 15N axial static pull for 15s without breakage and shedding.
5.16 protective cover
Each of its imports and exports shall have protective sleeves that are not naturally detached and firm but are easy to remove.
5.17 RBC Collection Bag
The blood transfusion socket, transparency and color of the red blood cell collection bag should meet the requirements in GB 14232.1-2004.
6 Chemical requirements
6.1 Separation cup and piping system
6.1.1 Reducing substances
When tested according to C.2, the test solution and blank solution prepared according to C.1.3 consume potassium permanganate solution [c(KMnO4)=0.002mol/L]
The difference in volume should not exceed 2.0 mL.
6.1.2 Metal ions
When determined by atomic absorption spectrophotometry (AAS) or equivalent method, the total content of antimony, chromium, copper, lead, and tin in the extract should be
Not exceeding 1 μg/mL, the content of cadmium should not exceed 0.1 μg/mL.
When tested in accordance with C.3, the color present in the extract should not exceed the standard ...
Get Quotation: Click YY/T 1566.1-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1566.1-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1566.1-2017: Autologous blood processing devices for single use - Part 1: Blood cell recovery sets, centrifuge bowl type
YY/T 1566.1-2017
Autologous blood processing devices for single use--Part 1. Blood cell recoery sets, centrifuge bowl type
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Disposable autologous blood processing equipment
Part 1. Centrifugal Cup Blood Cell Harvester
Part 1. Bloodcelrecoerysets,centrifugebowltype
Published on.2017-07-17
2018-07-01 Implementation
The State Food and Drug Administration issued
Directory
Preface III
Introduction IV
1 Range 1
2 Normative references 1
3 Terms and Definitions 1
4 Structure 3
5 Physical requirements 3
5.1 Appearance 3
5.2 Particle Pollution 3
5.2.1 Separation Cup 3
5.2.2 Piping System 3
5.2.3 RBC collection bag 3
5.3 Sealing 3
5.4 Connection strength 4
5.5 Frictional heat 4
5.6 Noise 4
5.7 Residual amount 4
5.8 color standard 4
5.9 Puncher 4
5.10 Dripping Bucket 4
5.11 Flow Regulator 4
5.12 Pump tube elasticity 4
5.13 limit card (if any) 4
5.14 Fixture 5
5.15 Interface 5
5.16 protective cover 5
5.17 RBC collection bag 5
6 Chemical Requirements 5
6.1 Separation Cup and Piping System 5
6.1.1 Reducing substance 5
6.1.2 Metal Ions 5
6.1.3 pH 5
6.1.4 Evaporation residue 5
6.1.5 UV absorbance 5
6.2 RBC collection bag 5
6.3 Ethylene Oxide Residue 5
7 Biological Requirements 6
7.1 Biocompatibility 6
7.2 Aseptic 6
7.3 Bacterial endotoxin 6
8 type inspection 6
9 Signs 6
9.1 General Clause 6
9.2 Single Pack Logo 6
9.3 Shelf Packing Mark 6
10 Packing 7
Appendix A (Informative) Note 8
Appendix B (Normative Appendix) Physical Test 10
Appendix C (Normative) Chemical Test 12
Reference 14
Figure 1 Schematic diagram of example 2
Foreword
YY/T 1566 "Disposable Autologous Blood Processing Apparatus" consists of the following components.
--- Part 1. Centrifugal cup blood cell harvester;
--- Part 2. Blood collection and filtration device (blood reservoir);
This section is Part 1 of YY/T 1566.
This section was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This part is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
This section mainly drafted by. Beijing Jingjing Medical Equipment Co., Ltd., Beijing Wandong Kangyuan Technology Development Co., Ltd., Shandong Province Medical
Device Product Quality Inspection Center.
The main drafters of this section. Zhang Mingli, Zhang Qing, Liu Ye, Yan Chunliang, Sun Lingyan.
introduction
As a disposable autologous blood processing device, a centrifugal cup blood cell recovery device is required to be used together with an autologous blood recovery device. Used outside
When a surgical or traumatic bleeding occurs, the patient's blood is recovered, blood components are separated, washed, replaced, etc., and finally collected into the red blood cells.
Collect the bag for return to the patient. ANSI/AAMIAT6.2013 Clinical Application of Autologous Blood Transfer Device for Autologous Blood Refusion
Please refer to Appendix A for information.
Disposable autologous blood processing equipment
Part 1. Centrifugal Cup Blood Cell Harvester
1 Scope
This part of YY/T 1566 specifies the requirements for a centrifuged cup-type blood cell recovery device used in surgery. It is a one-time use product.
Products to ensure the safety of use with autologous blood recovery machines.
This section applies to centrifuge cups that are intended to be used in conjunction with other autologous blood processing devices and are intended for use with autologous blood recovery equipment.
Blood cell recovery product mainly consists of a pipeline system, a separation cup, a red blood cell collection bag and a waste liquid bag, excluding the blood collection and filtration device
(Blood reservoir).
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 6682 Analysis Laboratory Water Specifications and Test Methods
GB 8368 Single-use infusion set gravity infusion
GB 14232.1-2004 Human blood and blood components Bag-type plastic containers Part 1. Traditional blood bags
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 14233.2 Medical infusion, blood transfusion, injection equipment inspection methods Part 2. Biological test methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB/T 19633.1 Packaging for terminally sterilized medical devices Part 1. Requirements for materials, sterile barrier systems, and packaging systems
YY/T 0466.1 Medical Devices Symbols for Labeling, Marking, and Providing Information on Medical Devices Part 1. General Requirements
YY 0584-2005 Centrifugal Cup Blood Composition Separator
YY/T 0615.1 Requirements for Labeling “Aseptic” Medical Devices Part 1. Requirements for Final Sterilization Medical Devices
ISO 7000 Device Graphic Symbols Registered Symbols (Graphicalsymbolsforuseonequipment-Indexand
Synopsis)
3 Terms and Definitions
The following terms and definitions apply to this document.
3.1
Autologous blood autologousblood
Collect and process the blood of a certain patient and return it to the patient's blood.
3.2
Blood reservoir reservoir
A filter storage container that can filter out blood debris or various blood constituent aggregates.
a) Autologous blood collection and anticoagulant introduction lines
b) Autogenous vacuum suction line
c) Autologous blood processing lines
Explanation.
1 ---Thrors;
2 --- drop bucket;
3 --- flow regulators;
4 --- suction pipe joints;
5 --- double tubes (blood collection tube and anti-condensation tube);
6 --- puncture protector;
7 --- Red blood cell collection bag;
8 --- Blood transfusion socket;
9 --- RBC collection bag connecting pipe;
10 --- pump tube;
11--- Blood reservoir connector (optional);
12---Protection cap;
13---pipe clamps;
14 --- separation cup;
15 --- separation cup connecting pipe;
16--- waste bag;
17--- suction tube.
Figure 1 Schematic diagram
4 Structure
A typical centrifugal cup blood cell recovery device consists of a tubing system (including a separate cup connecting tube, a double tube, a red blood cell collection bag connecting tube, a pump tube,
Auto blood pressure suction tube (optional), separation cup, red cell collection bag, and waste bag. Figure 1 shows a common structure
Intent example.
5 Physical requirements
5.1 Appearance
5.1.1 The cup body and piping system of the separation cup should be transparent or sufficiently transparent to detect water and normal or corrected vision when bubbles pass through.
Air interface.
5.1.2 The surface of the cup body of the separation cup should be smooth and clean. Visual inspection and feel should not cause obvious defects such as spots, impurities, scratches, and burrs.
5.1.3 The piping system shall be plasticized, unobstructed, and free of kinks.
5.1.4 The red blood cell collection bag and the waste bag bag body should have no obvious impurities, spots, bubbles, the inner and outer surfaces should be flat, the inner wall should not adhere, and the heat sealing line should be
Transparent, uniform, hanging holes should be regular. The outer surface of the logo should be clear and easy to identify.
5.1.5 The interface of the separation cup, clamps, and other components should be smooth and free from burrs.
5.2 Particle Pollution
5.2.1 separation cup
When measured in accordance with B.1, the number of particles in 15 μm to 25 μm in the.200 mL eluent should not exceed 6.00 particles/mL, which is greater than 25 μm.
The particles should not exceed 3.00 particles/mL.
5.2.2 Piping System
When measured in accordance with B.2, the number of particles 15 μm to 25 μm on the surface area per square centimeter should not exceed 1.00, greater than 25 μm.
The number of particles should not exceed 0.50.
5.2.3 RBC collection bag
The production of red blood cell collection bags should avoid particulate contamination.
When tested in accordance with the provisions of B.3, red blood cell collection bag should have no visible particles.
Note. Work is under way to establish limits on the number and size of particles. At present, the limits and test methods given in pharmacopoeias can be used (such as the European Pharmacopoeia
The limits and methods of the prescribed formulation).
5.3 Sealing
5.3.1 Separation cup The separation cup shall be free from leakage during the particle contamination test of the separation cup according to B.1.
Note. The sealability of the separation cup and the particulate contamination test are combined.
5.3.2 Close the other ends of the pipelines in the pipeline system, leaving only one end to pass 50kPa air pressure for 15s, underwater inspection should be no
Continuous bubble leaks.
5.3.3 Fill the RBC collection bag with GB/T 6682 water to nominal capacity and seal it. Then put the red blood cell collection bag
Extruded between the two plates, under the conditions of 23 °C ± 5 °C, so that the internal pressure rises above atmospheric pressure 50kPa, for 10min, should be
No leakage.
5.4 Connection strength
5.4.1 The joints (excluding the protective sleeve) of the centrifugal cup blood cell recovery device shall be able to withstand the static axial tension of 15N for 15s.
No breakage and shedding.
5.4.2 Holes or other suspension devices on the red blood cell collection bag should be able to withstand a static axial tension of 10N for 60 minutes without breaking.
5.4.3 Fill the waste bag with water to the maximum mark line, and suspend it for 2 hours under normal use conditions. The suspension device of the waste bag should be continuously
Cracked, and the bag body should have no leakage.
5.5 Frictional heat
When the separation cup is tested according to B.4, the water temperature in the cup should not exceed 37°C.
5.6 Noise
When the separation cup is operated at 5600r/min on the used autologous blood recovery machine, there should be no obvious swing, and the separation cups are at the front, rear, left and right sides.
When the center noise is measured with a sound level meter (A weighting) at a distance of 1m, the maximum noise of the separation cup should not be exceeded when the maximum noise of the main engine does not exceed 60dB.
More than 70dB.
Note. When the manufacturer gives the centrifuge speed, the test is carried out according to the centrifugal speed given by the manufacturer, but not given according to the centrifugal speed of 5600 r/min.
Test.
5.7 Residual amount
When the separation cup is tested according to B.5 or other equivalent methods, the residual amount in the cup should not exceed 22 mL.
5.8 color standard
Each section of the separation cup connecting line (not including the part connected to the waste bag) should adopt different colors of color to provide users with
For identification.
5.9 Puncher
5.9.1 After inserting a blood bag socket conforming to GB 14232.1-2004, the puncture device shall be able to withstand a static tension of 15N for 15 seconds.
5.9.2 When tested in accordance with 5.3 of GB 14232.1-2004, the connection between the puncture device and the blood bag socket shall be free from leakage.
5.10 Dripping Bucket
Droplets should be able to introduce anticoagulant liquids or other liquids into the piping system by means of elastic forces.
5.11 Flow Regulator
Flow regulators should comply with the provisions of GB 8368.
5.12 Pump Tube 1) Elasticity
Pipeline pump tube part should have good elasticity, when the water temperature is 23 °C ± 2 °C, according to A.6 test, the flow rate decreases after 1h
The rate should be less than 5%.
5.13 limit card 2) (if any)
5.13.1 Limit cards should have color identification. The limit card should be adapted to the upper limit device of the autogenous blood recovery machine.
1) Pump tubing refers to the hose installed in the peristaltic pump of the host.
2) The limit card is a plastic part that is installed on the pump tube to limit the displacement of the pump tube and is an optional accessory.
5.13.2 The limit card should be firmly bonded to the pump tube and it is not easy to fall off and shift manually.
5.14 Fixture
When the fixture is closed, it should be able to block the flow of 50kPa gas and liquid, and after opening, it will not damage the pipeline, to ensure the liquid flow.
5.15 Interface
If there is an interface on the piping system that is intended to be connected to the blood reservoir piping, it shall be provided with its associated or manufacturer-specified blood reservoir interface.
Match, according to the instructions for use, should be subjected to 15N axial static pull for 15s without breakage and shedding.
5.16 protective cover
Each of its imports and exports shall have protective sleeves that are not naturally detached and firm but are easy to remove.
5.17 RBC Collection Bag
The blood transfusion socket, transparency and color of the red blood cell collection bag should meet the requirements in GB 14232.1-2004.
6 Chemical requirements
6.1 Separation cup and piping system
6.1.1 Reducing substances
When tested according to C.2, the test solution and blank solution prepared according to C.1.3 consume potassium permanganate solution [c(KMnO4)=0.002mol/L]
The difference in volume should not exceed 2.0 mL.
6.1.2 Metal ions
When determined by atomic absorption spectrophotometry (AAS) or equivalent method, the total content of antimony, chromium, copper, lead, and tin in the extract should be
Not exceeding 1 μg/mL, the content of cadmium should not exceed 0.1 μg/mL.
When tested in accordance with C.3, the color present in the extract should not exceed the standard ...
Share