1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1571-2017 English PDF (YYT1571-2017)
YY/T 1571-2017 English PDF (YYT1571-2017)
Regular price
$320.00 USD
Regular price
Sale price
$320.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1571-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1571-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1571-2017: Tissue engineering medical device products - Sodium hyaluronate
YY/T 1571-2017
Tissue engineering medical device products-Sodium hyaluronate
ICS 11.040.40
C45
People's Republic of China pharmaceutical industry standards
Replacing YY/T 0606.9-2007
Tissue engineering medical equipment products sodium hyaluronate
2017-05-02 released
2018-04-01 implementation
State Food and Drug Administration released
Directory
Foreword Ⅲ
1 range 1
2 Normative references 1
3 Terms and definitions 1
4 Categories 2
5 Requirements 2
6 test method 4
7 logo 6
8 packaging, transportation and storage 7
Appendix A (Normative) Determination of sodium hyaluronate content 8
Appendix B (Normative) Determination of protein content 10
Appendix C (Normative) Determination of ethanol residues (headspace gas chromatography) 12
Appendix D (Informative) Determination of quaternary ammonium salt (cetyl pyridinium chloride) residues 14
Appendix E (Normative) Determination of weight average molecular weight and molecular weight distribution coefficient 16
References 17
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY/T 0606.9-2007 "tissue engineering medical products Part 9. Sodium hyaluronate." With YY/T 0606.9-
2007 compared to the main technical changes are as follows.
--- Standard name changed to "tissue engineering medical equipment products sodium hyaluronate";
--- Removed YY/T 16886 series of normative references (YY/T 16886.1 reserved only) (see Chapter 2,.2007 Edition
chapter 2);
--- Increasing the normative references YY/T 0771.1 ~ 0771.3 animal-derived medical devices (see Chapter 2);
--- Modify the normative references in the "People's Republic of China Pharmacopoeia" version number (see Chapter 2,.2007 edition
chapter 2);
--- Modified sodium hyaluronate formula (structural unit) (see 3.2,.2007 edition of 3.2);
--- Modified sodium hyaluronate content requirements and test methods (see 5.3,6.3 and Appendix A,.2007 Edition 5.3, 6.3 and
Appendix A);
--- Modify the intrinsic viscosity of the requirements and test methods formula molecular weight symbol M (see 5.5, 6.5,.2007 edition 5.5,
6.5);
--- Remove the dynamic viscosity requirements and test methods (see.2007 version 5.6,6.6);
--- Modify the test method of protein content (see 6.6 and Appendix B, the.2007 edition of 6.7 and Appendix B);
--- Increased nucleic acid requirements and test methods (see 5.7 and 6.7);
--- Modify the test method for heavy metal content (see 6.8,.2007 edition of 6.8);
--- Revised ethanol residue requirements and test methods (see 5.9,6.9 and Appendix C,.2007 edition of 5.9,6.9 and Appendix C);
--- Remove the ash requirements and test methods (see.2007 edition 5.11,6.11);
--- Remove the UV absorption requirements and test methods (see.2007 version 5.12,6.12);
--- Modify the dry matter content requirements and test methods (see 5.10, 6.10,.2007 edition of 5.10,6.10);
--- Increasing quaternary ammonium residue requirements and test methods (see 5.11,6.11 and Appendix D);
--- Increasing sulfated mucopolysaccharide requirements and test methods (see 5.12 and 6.12);
--- Increasing the iron content requirements and test methods (see 5.13 and 6.13);
--- Increasing chloride requirements and test methods (see 5.14 and 6.14);
--- Increasing the weight average molecular weight and molecular weight distribution coefficient requirements and test methods (see 5.15, 6.15 and Appendix E);
--- Increasing the clarity of the solution and the color requirements and test methods (see 5.16 and 6.16);
--- Increase the microbial limit requirements and test methods (see 5.18,6.18);
--- Modified bacterial endotoxin limit requirements and test methods (see 5.19.6.19,.2007 edition of 5.14,6.14);
--- Modify the raw material safety requirements and test methods (see 5.20, 6.20, 6.11,.2007 edition of 5.15,6.15);
--- Deleted the biological evaluation of specific requirements and test methods, leaving only the general (see 5.11,.2007 edition 5.16,6.16);
--- Removed the seventh chapter inspection rules (see the.2007 edition of Chapter 7).
--- Removed Appendix D background information (see.2007 edition of Appendix D);
--- Added reference "animal-derived medical device product registration data reporting guidelines" and the European Pharmacopoeia (see references);
--- Modify the reference number ASTMF2347-03 version (see references,.2007 edition of the reference).
Please note that some of this document may be patentable. The issuing agencies of this document do not bear the responsibility of identifying these patents.
This standard proposed by the State Food and Drug Administration.
This standard by the National Surgical Implants and Orthopedic Instruments Standardization Technical Committee Organization Engineering Medical Devices Products Technical Committee
Focused.
This standard was drafted unit. Huaxi Furui Da Biomedical Co., Ltd., China Institute of Food and Drug Control, Shanghai Qisheng biological agents have
Limited company.
The main drafters of this standard. Guo Xueping, Mu Shu E, Jiang Lixia, Xu Liming, Wei Changzheng, Wang Xiujuan, Shao Anliang.
This standard replaces the standards previously issued as.
--- YY/T 0606.9-2007.
Tissue engineering medical equipment products sodium hyaluronate
1 Scope
This standard specifies the requirements for surgical implants and tissue engineering medical devices sodium hyaluronate, test methods.
This standard applies to the preparation of tissue engineering medical device products and scaffolds sodium hyaluronate.
2 Normative references
The following documents for the application of this document is essential. For dated references, only the dated version applies to this article
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 191 Packaging - Pictorial signs
GB/T 14518 adhesive pH measurement
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation and test in risk management process (GB/T 16886.1-
2011, ISO 10993-1.2009, IDT)
GB 18278 (all parts) Health care products Sterilization Damp heat
GB 18279 (all parts) Health care products Sterilized ethylene oxide
GB 18280 (all parts) Health care products Sterilization radiation
YY/T 0313 medical polymer packaging and product manufacturers to provide information requirements
YY/T 0606.25 Tissue engineering medical products - Part 25. Determination of DNA residue of animal-derived biomaterials - Fluorescent staining
Color method
YY/T 0771.1 Animal-derived medical devices Part 1. Risk management applications (YY/T 0771.1-2009, ISO 22442-1.
2007, IDT)
YY/T 0771.2 animal-derived medical devices Part 2. Source, collection and disposal control (YY/T 0771.2-2009,
ISO 22442-2.2007, IDT)
YY/T 0771.3 Animal-derived medical devices - Part 3. Removal and inactivation of viruses and transmissible spongiform encephalopathies (TSEs)
Confirmation (YY/T 0771.3-2009, ISO 22442-3.2007, IDT)
"The People's Republic of China Pharmacopoeia" (2015 edition, four)
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Hyaluronic acid hyaluronicacid
The disaccharide repeating structural unit consisting of D-glucuronic acid and N-acetyl-D-glucosamine linked through β- (1-3) glycosidic bonds
Linear polysaccharide. Each disaccharide unit is linked to the other by beta- (1-4) glycosidic bonds.
3.2
Sodium hyaluronate
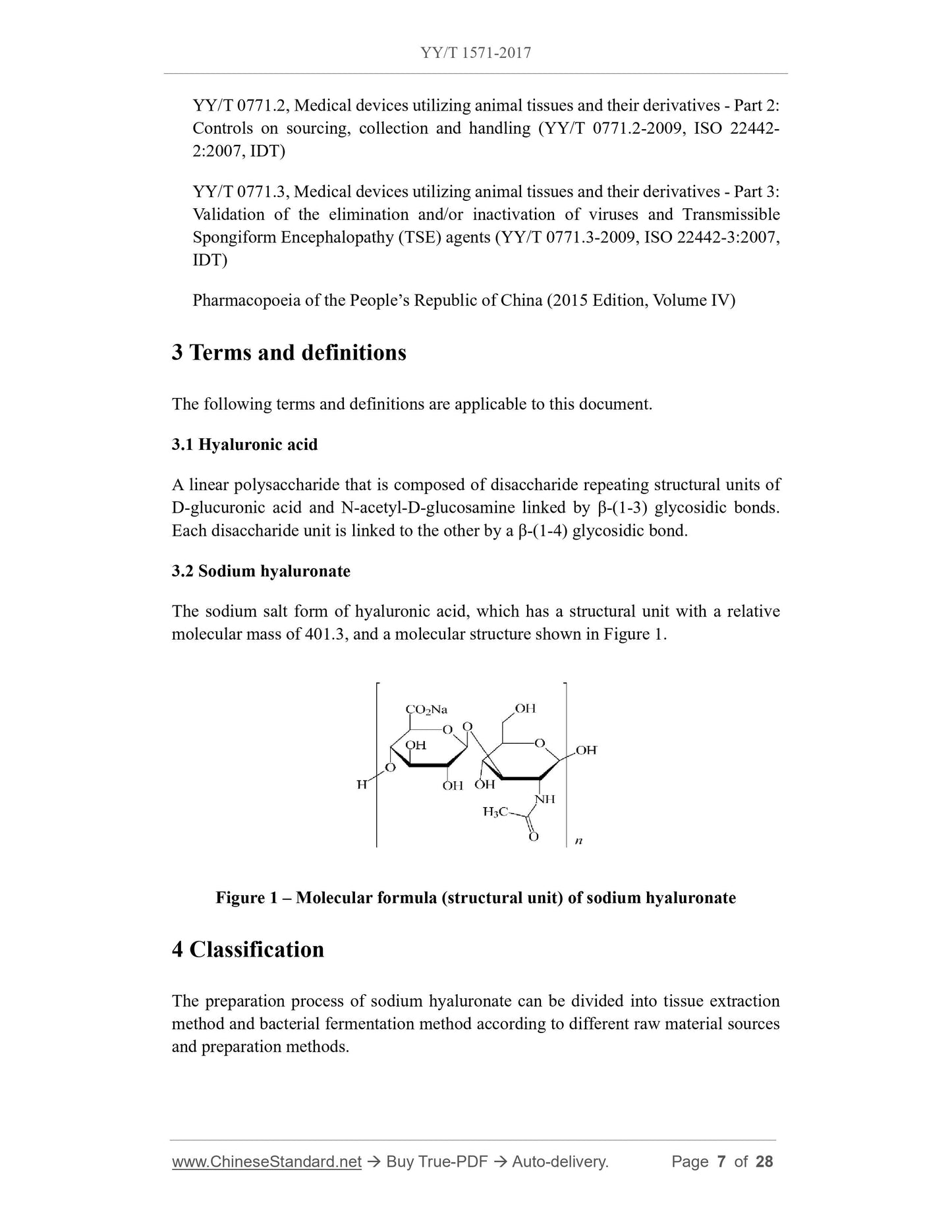
The sodium salt form of hyaluronic acid, the relative molecular mass of the structural unit is 401.3, the molecular structural formula is shown in FIG. 1.
Get Quotation: Click YY/T 1571-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1571-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1571-2017: Tissue engineering medical device products - Sodium hyaluronate
YY/T 1571-2017
Tissue engineering medical device products-Sodium hyaluronate
ICS 11.040.40
C45
People's Republic of China pharmaceutical industry standards
Replacing YY/T 0606.9-2007
Tissue engineering medical equipment products sodium hyaluronate
2017-05-02 released
2018-04-01 implementation
State Food and Drug Administration released
Directory
Foreword Ⅲ
1 range 1
2 Normative references 1
3 Terms and definitions 1
4 Categories 2
5 Requirements 2
6 test method 4
7 logo 6
8 packaging, transportation and storage 7
Appendix A (Normative) Determination of sodium hyaluronate content 8
Appendix B (Normative) Determination of protein content 10
Appendix C (Normative) Determination of ethanol residues (headspace gas chromatography) 12
Appendix D (Informative) Determination of quaternary ammonium salt (cetyl pyridinium chloride) residues 14
Appendix E (Normative) Determination of weight average molecular weight and molecular weight distribution coefficient 16
References 17
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY/T 0606.9-2007 "tissue engineering medical products Part 9. Sodium hyaluronate." With YY/T 0606.9-
2007 compared to the main technical changes are as follows.
--- Standard name changed to "tissue engineering medical equipment products sodium hyaluronate";
--- Removed YY/T 16886 series of normative references (YY/T 16886.1 reserved only) (see Chapter 2,.2007 Edition
chapter 2);
--- Increasing the normative references YY/T 0771.1 ~ 0771.3 animal-derived medical devices (see Chapter 2);
--- Modify the normative references in the "People's Republic of China Pharmacopoeia" version number (see Chapter 2,.2007 edition
chapter 2);
--- Modified sodium hyaluronate formula (structural unit) (see 3.2,.2007 edition of 3.2);
--- Modified sodium hyaluronate content requirements and test methods (see 5.3,6.3 and Appendix A,.2007 Edition 5.3, 6.3 and
Appendix A);
--- Modify the intrinsic viscosity of the requirements and test methods formula molecular weight symbol M (see 5.5, 6.5,.2007 edition 5.5,
6.5);
--- Remove the dynamic viscosity requirements and test methods (see.2007 version 5.6,6.6);
--- Modify the test method of protein content (see 6.6 and Appendix B, the.2007 edition of 6.7 and Appendix B);
--- Increased nucleic acid requirements and test methods (see 5.7 and 6.7);
--- Modify the test method for heavy metal content (see 6.8,.2007 edition of 6.8);
--- Revised ethanol residue requirements and test methods (see 5.9,6.9 and Appendix C,.2007 edition of 5.9,6.9 and Appendix C);
--- Remove the ash requirements and test methods (see.2007 edition 5.11,6.11);
--- Remove the UV absorption requirements and test methods (see.2007 version 5.12,6.12);
--- Modify the dry matter content requirements and test methods (see 5.10, 6.10,.2007 edition of 5.10,6.10);
--- Increasing quaternary ammonium residue requirements and test methods (see 5.11,6.11 and Appendix D);
--- Increasing sulfated mucopolysaccharide requirements and test methods (see 5.12 and 6.12);
--- Increasing the iron content requirements and test methods (see 5.13 and 6.13);
--- Increasing chloride requirements and test methods (see 5.14 and 6.14);
--- Increasing the weight average molecular weight and molecular weight distribution coefficient requirements and test methods (see 5.15, 6.15 and Appendix E);
--- Increasing the clarity of the solution and the color requirements and test methods (see 5.16 and 6.16);
--- Increase the microbial limit requirements and test methods (see 5.18,6.18);
--- Modified bacterial endotoxin limit requirements and test methods (see 5.19.6.19,.2007 edition of 5.14,6.14);
--- Modify the raw material safety requirements and test methods (see 5.20, 6.20, 6.11,.2007 edition of 5.15,6.15);
--- Deleted the biological evaluation of specific requirements and test methods, leaving only the general (see 5.11,.2007 edition 5.16,6.16);
--- Removed the seventh chapter inspection rules (see the.2007 edition of Chapter 7).
--- Removed Appendix D background information (see.2007 edition of Appendix D);
--- Added reference "animal-derived medical device product registration data reporting guidelines" and the European Pharmacopoeia (see references);
--- Modify the reference number ASTMF2347-03 version (see references,.2007 edition of the reference).
Please note that some of this document may be patentable. The issuing agencies of this document do not bear the responsibility of identifying these patents.
This standard proposed by the State Food and Drug Administration.
This standard by the National Surgical Implants and Orthopedic Instruments Standardization Technical Committee Organization Engineering Medical Devices Products Technical Committee
Focused.
This standard was drafted unit. Huaxi Furui Da Biomedical Co., Ltd., China Institute of Food and Drug Control, Shanghai Qisheng biological agents have
Limited company.
The main drafters of this standard. Guo Xueping, Mu Shu E, Jiang Lixia, Xu Liming, Wei Changzheng, Wang Xiujuan, Shao Anliang.
This standard replaces the standards previously issued as.
--- YY/T 0606.9-2007.
Tissue engineering medical equipment products sodium hyaluronate
1 Scope
This standard specifies the requirements for surgical implants and tissue engineering medical devices sodium hyaluronate, test methods.
This standard applies to the preparation of tissue engineering medical device products and scaffolds sodium hyaluronate.
2 Normative references
The following documents for the application of this document is essential. For dated references, only the dated version applies to this article
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 191 Packaging - Pictorial signs
GB/T 14518 adhesive pH measurement
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation and test in risk management process (GB/T 16886.1-
2011, ISO 10993-1.2009, IDT)
GB 18278 (all parts) Health care products Sterilization Damp heat
GB 18279 (all parts) Health care products Sterilized ethylene oxide
GB 18280 (all parts) Health care products Sterilization radiation
YY/T 0313 medical polymer packaging and product manufacturers to provide information requirements
YY/T 0606.25 Tissue engineering medical products - Part 25. Determination of DNA residue of animal-derived biomaterials - Fluorescent staining
Color method
YY/T 0771.1 Animal-derived medical devices Part 1. Risk management applications (YY/T 0771.1-2009, ISO 22442-1.
2007, IDT)
YY/T 0771.2 animal-derived medical devices Part 2. Source, collection and disposal control (YY/T 0771.2-2009,
ISO 22442-2.2007, IDT)
YY/T 0771.3 Animal-derived medical devices - Part 3. Removal and inactivation of viruses and transmissible spongiform encephalopathies (TSEs)
Confirmation (YY/T 0771.3-2009, ISO 22442-3.2007, IDT)
"The People's Republic of China Pharmacopoeia" (2015 edition, four)
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Hyaluronic acid hyaluronicacid
The disaccharide repeating structural unit consisting of D-glucuronic acid and N-acetyl-D-glucosamine linked through β- (1-3) glycosidic bonds
Linear polysaccharide. Each disaccharide unit is linked to the other by beta- (1-4) glycosidic bonds.
3.2
Sodium hyaluronate
The sodium salt form of hyaluronic acid, the relative molecular mass of the structural unit is 401.3, the molecular structural formula is shown in FIG. 1.
Share