1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1691-2020 English PDF (YY/T1691-2020)
YY/T 1691-2020 English PDF (YY/T1691-2020)
Regular price
$145.00 USD
Regular price
Sale price
$145.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1691-2020 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1691-2020
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1691-2020: Dentistry--Torque transmitter for handpieces
YY/T 1691-2020
Dentistry--Torque transmitter for handpieces
ICS 11.060.25
C33
People's Republic of China Pharmaceutical Industry Standard
Dental handpiece torque transmitter
(ISO 17509.2016,MOD)
2020-02-21 released
2021-06-01 implementation
Issued by the State Drug Administration
Preface
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard uses the redrafting method to modify and adopt ISO 17509.2016 "Dental Handpiece Torque Transmitter" (English version).
Compared with ISO 17509.2016, the main technical differences between this standard are as follows.
---The ISO preface is deleted.
---According to the Chinese language convention, the order of paragraphs in "Scope" has been adjusted.
---Regarding normative reference documents, this standard has been adjusted to adapt to my country's technical conditions and to facilitate the implementation of this standard.
The situation is collectively reflected in Chapter 2 "Normative Reference Documents", and the specific adjustments are as follows.
● Replace ISO 6507-1 with the modified GB/T 4340.1 (see 6.3);
● Replace ISO 3274 with the equivalent GB/T 6062 (see 6.1);
● Replace ISO 1942 (all parts) with GB/T 9937 (all parts) (see Chapter 3), the difference between each part of the two standards
The degree of consistency is as follows.
● GB/T 9937.1-2008 Oral Vocabulary Part 1.Basic and Clinical Terminology (ISO 1942-1.1989,
IDT);
● GB/T 9937.2-2008 Dental Vocabulary Part 2.Dental Materials (ISO 1942-2.1989, IDT);
● GB/T 9937.3-2008 Dental Vocabulary Part 3.Dental Devices (ISO 1942-3.1989, IDT);
● GB/T 9937.4-2005 Dental Terminology Part 4.Dental Equipment (ISO 1942-4.1989, IDT);
● GB/T 9937.5-2008 Oral Vocabulary Part 5.Terminology Related to Testing (ISO 1942-5.1989,
IDT);
● Replace ISO 4288 with the equivalent GB/T 10610 (see 6.1);
● Replace ISO 1797-1.1992 with the modified YY/T 0967.1-2015 (see 6.1, 11.3.1);
● Replace IEC 62366-1 with the equivalent YY/T 1474 (see Chapter 5, 6.2);
● Replace ISO 13504.2012 with the equivalent YY/T 1486-2016 (see Chapter 3 to Chapter 10);
● Replace ISO 16443 with the equivalent YY/T 1619 (see Chapter 3);
● ISO 10664 is deleted.
---Added "Note. Figure 1~Figure 4 are examples" in "6.1 Dimensions", and refer to Figure 1~Figure 4 in the standard text.
--- "The test is carried out in accordance with the method specified in 11.1" in "6.1 Dimensions" is amended to "the test shall be conducted in accordance with the method specified in 11.1 and 11.2
To make the test method better meet the technical requirements.
Please note that certain contents of this document may involve patents. The issuing agency of this document is not responsible for identifying these patents.
This standard was proposed by the State Drug Administration.
This standard is organized by the National Dental Materials and Equipment Standardization Technical Committee Dental Equipment and Equipment Sub-Technical Committee (SAC/TC99
SC1) Centralized.
Drafting organizations of this standard. Guangdong Medical Device Quality Supervision and Inspection Institute, Sino Medical Device Group Co., Ltd., Foshan Biying Medical
Equipment Co., Ltd., Shenzhen Suhang Technology Development Co., Ltd.
The main drafters of this standard. Li Wei, Liu Zhiwei, Chen Zhuoqiang, Zhao Lijun, Zeng Wenbin, Wang Zhong.
introduction
This standard is based on YY/T 1486-2016 "General Requirements for Dental Implant Devices and Related Auxiliary Devices in Dentistry".
Describes an auxiliary device, which is used to insert a dental handpiece and transfer torque from the dental handpiece to the dental implant or its connection
part.
Dental handpiece torque transmitter
1 Scope
This standard specifies the terms and definitions, classification, expected performance, performance attributes, performance evaluation, production, and repeated processing tolerance of torque transmitters.
Performance, information and test methods provided by the manufacturer. This torque transmitter is used as an auxiliary device to connect to a dental handpiece and used in dental implants
Place the oral implant and its connecting parts in the craniofacial area for further operations.
This standard applies to torque transmitters for implanting or removing implants in the patient’s mouth.
Functional power drive system, this standard does not include dental implants or components connected to dental implants.
This standard does not apply to the power drive system itself.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 4340.1 Vickers hardness test of metallic materials Part 1.Test method (GB/T 4340.1-2009, ISO 6507-
1.2005, MOD)
GB/T 6062 Product Geometric Technical Specification (GPS) Surface structure contour method contact (stylus) type instrument nominal characteristics
(GB/T 6062-2009, ISO 3274.1996, IDT)
GB/T 9937 (all parts) oral vocabulary [ISO 1942 (all parts)]
GB/T 10610 Geometrical Technical Specifications for Products (GPS) The rules and methods of surface structure profile method for evaluating surface structure
(GB/T 10610-2009, ISO 4288.1996, IDT)
YY/T 0967.1-2015 Dental rotating instrument rod Part 1.Metal rod (ISO 1797-1.1992, MOD)
YY/T 1474 Application of medical device usability engineering to medical devices (YY/T 1474-2016, IEC 62366.2007,
IDT)
YY/T 1486-2016 General requirements for dental implant equipment and related auxiliary equipment (ISO 13504.2012,
IDT)
YY/T 1619 Dental implant system and related process terms (YY/T 1619-2018, ISO 16443.2014, IDT)
3 Terms and definitions
The following terms and definitions defined in GB/T 9937, YY/T 1486-2016, YY/T 1619, and are applicable to this document.
3.1
Auxiliary instruments for dental implants are non-invasive surgical instruments used for short-term use in direct or indirect contact with the human body during dental implant implantation and subsequent related treatments.
3.2
The torque transmitter is a non-invasive surgical instrument used to transmit torque from the dental handpiece to the dental implant or its connecting parts.
4 categories
Torque transmitters are classified according to 4.1~4.3 in YY/T 1486-2016, see Table 1.
Table 1 Torque transmitter classification
YY/T 1486-2016 clause classification requirements category description
4.1 Intended use type 1 energized or powered devices
4.2 Organizational contact 3 types of unorganized contact
4.3 Reuse the first group multiple times
5 Expected performance
5a) and 5b) in YY/T 1486-2016 and YY/T 1474 apply.
6 Performance attributes
Chapter 6 of YY/T 1486-2016 is applicable.
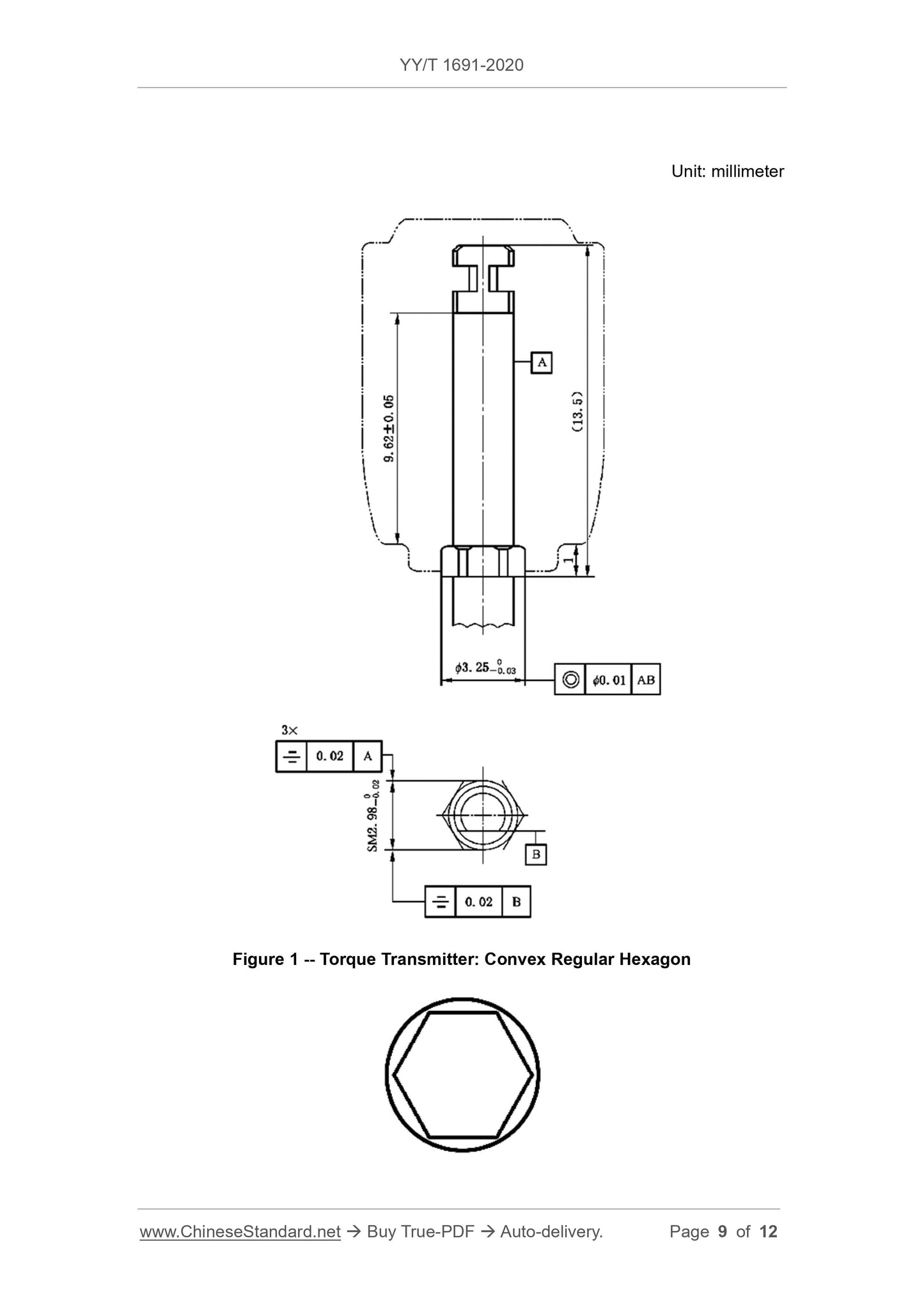
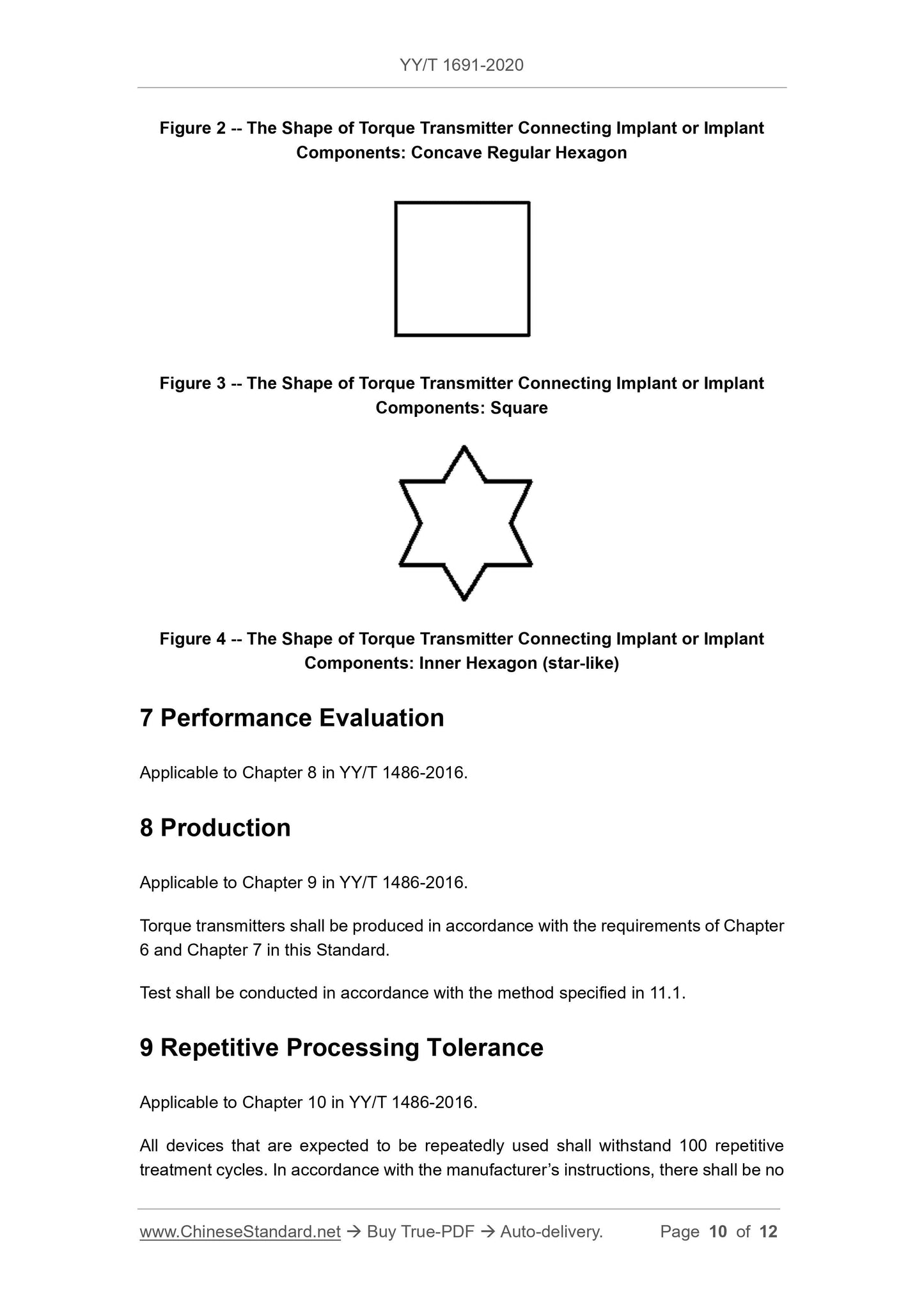
6.1 Dimensions
The size and tolerance of the rod should meet the requirements of the type 1 rod in YY/T 0967.1-2015.The shape and size of the type 1 rod and the head are manufactured by
Quotient.
Note. Figures 1 to 4 are examples.
The surface roughness should meet the requirements of YY/T 0967.1-2015, according to the methods specified in GB/T 6062 and GB/T 10610
test.
The test is carried out in accordance with the methods specified in 11.1 and 11.2.
6.2 Performance
The torque transmitter connected to a suitable dental handpiece should be designed to transmit a torque of at least 0.8 N·m to the dental implant.
The shape and size of the part of the implant or connecting part connected to the torque transmitter should at least be included in the label information.
The test is carried out in accordance with the method specified in 11.3.
Note. The measured value is a technical limit rather than a medical description.
The structure of the torque transmitter should be in accordance with the requirements of YY/T 1474, providing safe and reliable operation in its connection with the mobile phone.
6.3 Material selection
Chapter 7 of YY/T 1486-2016 applies.
The manufacturer of the torque transmitter should use the materials specified in Appendix A of YY/T 1486-2016.
The hardness of steel rods is tested by the method specified in GB/T 4340.1, and the Vickers hardness should not be less than 500HV5.
7 Performance evaluation
Chapter 8 of YY/T 1486-2016 is applicable.
8 Production
Chapter 9 of YY/T 1486-2016 applies.
The torque transmitter shall be produced in accordance with the requirements of Chapter 6 and Chapter 7 of this standard.
The test is carried out in accordance with the method specified in 11.1.
9 Repeated treatment tolerance
Chapter 10 of YY/T 1486-2016 applies.
All devices that are expected to be used multiple times should withstand a repeated treatment cycle of 100 times, and according to the manufacturer's instructions, there should be no performance
Signs of decline or corrosion.
The repeated treatment cycle should include the recommended cleaning, disinfection and sterilization methods.
10 Information provided by the manufacturer
Chapter 11 of YY/T 1486-2016 applies.
The shape and size of the part of the implant or connecting part connected to the torque transmitter should at least be included in the label information.
Where appropriate, the manufacturer's name or registered trademark should be marked on the device and related auxiliary devices.
11 Test method
11.1 Technical documents for inspection products
Check the product technical documents to determine whether it meets the requirements.
11.2 Dimensions
Through visual inspection and the use of suitable measuring instruments for measurement, to determine whether it meets the requirements of all dimensions described by the manufacturer.
11.3 Locked-rotor torque
11.3.1 Instruments
The instrument requirements are as follows.
a) The clamping system complies with YY/T 0967.1-2015.
b) Torque meter or dynamometer can measure torque in Newton meters with an accuracy of ±10%.
11.3.2 Procedure
The torque transmitter is inserted into the clamping system and fixed, the torque measuring device is connected to the outer end of the torque transmitter, and the torque meter is slowly rotated until it reaches the
When the maximum allowable torque is reached, no deformation should be observed.
Get Quotation: Click YY/T 1691-2020 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1691-2020
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1691-2020: Dentistry--Torque transmitter for handpieces
YY/T 1691-2020
Dentistry--Torque transmitter for handpieces
ICS 11.060.25
C33
People's Republic of China Pharmaceutical Industry Standard
Dental handpiece torque transmitter
(ISO 17509.2016,MOD)
2020-02-21 released
2021-06-01 implementation
Issued by the State Drug Administration
Preface
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard uses the redrafting method to modify and adopt ISO 17509.2016 "Dental Handpiece Torque Transmitter" (English version).
Compared with ISO 17509.2016, the main technical differences between this standard are as follows.
---The ISO preface is deleted.
---According to the Chinese language convention, the order of paragraphs in "Scope" has been adjusted.
---Regarding normative reference documents, this standard has been adjusted to adapt to my country's technical conditions and to facilitate the implementation of this standard.
The situation is collectively reflected in Chapter 2 "Normative Reference Documents", and the specific adjustments are as follows.
● Replace ISO 6507-1 with the modified GB/T 4340.1 (see 6.3);
● Replace ISO 3274 with the equivalent GB/T 6062 (see 6.1);
● Replace ISO 1942 (all parts) with GB/T 9937 (all parts) (see Chapter 3), the difference between each part of the two standards
The degree of consistency is as follows.
● GB/T 9937.1-2008 Oral Vocabulary Part 1.Basic and Clinical Terminology (ISO 1942-1.1989,
IDT);
● GB/T 9937.2-2008 Dental Vocabulary Part 2.Dental Materials (ISO 1942-2.1989, IDT);
● GB/T 9937.3-2008 Dental Vocabulary Part 3.Dental Devices (ISO 1942-3.1989, IDT);
● GB/T 9937.4-2005 Dental Terminology Part 4.Dental Equipment (ISO 1942-4.1989, IDT);
● GB/T 9937.5-2008 Oral Vocabulary Part 5.Terminology Related to Testing (ISO 1942-5.1989,
IDT);
● Replace ISO 4288 with the equivalent GB/T 10610 (see 6.1);
● Replace ISO 1797-1.1992 with the modified YY/T 0967.1-2015 (see 6.1, 11.3.1);
● Replace IEC 62366-1 with the equivalent YY/T 1474 (see Chapter 5, 6.2);
● Replace ISO 13504.2012 with the equivalent YY/T 1486-2016 (see Chapter 3 to Chapter 10);
● Replace ISO 16443 with the equivalent YY/T 1619 (see Chapter 3);
● ISO 10664 is deleted.
---Added "Note. Figure 1~Figure 4 are examples" in "6.1 Dimensions", and refer to Figure 1~Figure 4 in the standard text.
--- "The test is carried out in accordance with the method specified in 11.1" in "6.1 Dimensions" is amended to "the test shall be conducted in accordance with the method specified in 11.1 and 11.2
To make the test method better meet the technical requirements.
Please note that certain contents of this document may involve patents. The issuing agency of this document is not responsible for identifying these patents.
This standard was proposed by the State Drug Administration.
This standard is organized by the National Dental Materials and Equipment Standardization Technical Committee Dental Equipment and Equipment Sub-Technical Committee (SAC/TC99
SC1) Centralized.
Drafting organizations of this standard. Guangdong Medical Device Quality Supervision and Inspection Institute, Sino Medical Device Group Co., Ltd., Foshan Biying Medical
Equipment Co., Ltd., Shenzhen Suhang Technology Development Co., Ltd.
The main drafters of this standard. Li Wei, Liu Zhiwei, Chen Zhuoqiang, Zhao Lijun, Zeng Wenbin, Wang Zhong.
introduction
This standard is based on YY/T 1486-2016 "General Requirements for Dental Implant Devices and Related Auxiliary Devices in Dentistry".
Describes an auxiliary device, which is used to insert a dental handpiece and transfer torque from the dental handpiece to the dental implant or its connection
part.
Dental handpiece torque transmitter
1 Scope
This standard specifies the terms and definitions, classification, expected performance, performance attributes, performance evaluation, production, and repeated processing tolerance of torque transmitters.
Performance, information and test methods provided by the manufacturer. This torque transmitter is used as an auxiliary device to connect to a dental handpiece and used in dental implants
Place the oral implant and its connecting parts in the craniofacial area for further operations.
This standard applies to torque transmitters for implanting or removing implants in the patient’s mouth.
Functional power drive system, this standard does not include dental implants or components connected to dental implants.
This standard does not apply to the power drive system itself.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 4340.1 Vickers hardness test of metallic materials Part 1.Test method (GB/T 4340.1-2009, ISO 6507-
1.2005, MOD)
GB/T 6062 Product Geometric Technical Specification (GPS) Surface structure contour method contact (stylus) type instrument nominal characteristics
(GB/T 6062-2009, ISO 3274.1996, IDT)
GB/T 9937 (all parts) oral vocabulary [ISO 1942 (all parts)]
GB/T 10610 Geometrical Technical Specifications for Products (GPS) The rules and methods of surface structure profile method for evaluating surface structure
(GB/T 10610-2009, ISO 4288.1996, IDT)
YY/T 0967.1-2015 Dental rotating instrument rod Part 1.Metal rod (ISO 1797-1.1992, MOD)
YY/T 1474 Application of medical device usability engineering to medical devices (YY/T 1474-2016, IEC 62366.2007,
IDT)
YY/T 1486-2016 General requirements for dental implant equipment and related auxiliary equipment (ISO 13504.2012,
IDT)
YY/T 1619 Dental implant system and related process terms (YY/T 1619-2018, ISO 16443.2014, IDT)
3 Terms and definitions
The following terms and definitions defined in GB/T 9937, YY/T 1486-2016, YY/T 1619, and are applicable to this document.
3.1
Auxiliary instruments for dental implants are non-invasive surgical instruments used for short-term use in direct or indirect contact with the human body during dental implant implantation and subsequent related treatments.
3.2
The torque transmitter is a non-invasive surgical instrument used to transmit torque from the dental handpiece to the dental implant or its connecting parts.
4 categories
Torque transmitters are classified according to 4.1~4.3 in YY/T 1486-2016, see Table 1.
Table 1 Torque transmitter classification
YY/T 1486-2016 clause classification requirements category description
4.1 Intended use type 1 energized or powered devices
4.2 Organizational contact 3 types of unorganized contact
4.3 Reuse the first group multiple times
5 Expected performance
5a) and 5b) in YY/T 1486-2016 and YY/T 1474 apply.
6 Performance attributes
Chapter 6 of YY/T 1486-2016 is applicable.
6.1 Dimensions
The size and tolerance of the rod should meet the requirements of the type 1 rod in YY/T 0967.1-2015.The shape and size of the type 1 rod and the head are manufactured by
Quotient.
Note. Figures 1 to 4 are examples.
The surface roughness should meet the requirements of YY/T 0967.1-2015, according to the methods specified in GB/T 6062 and GB/T 10610
test.
The test is carried out in accordance with the methods specified in 11.1 and 11.2.
6.2 Performance
The torque transmitter connected to a suitable dental handpiece should be designed to transmit a torque of at least 0.8 N·m to the dental implant.
The shape and size of the part of the implant or connecting part connected to the torque transmitter should at least be included in the label information.
The test is carried out in accordance with the method specified in 11.3.
Note. The measured value is a technical limit rather than a medical description.
The structure of the torque transmitter should be in accordance with the requirements of YY/T 1474, providing safe and reliable operation in its connection with the mobile phone.
6.3 Material selection
Chapter 7 of YY/T 1486-2016 applies.
The manufacturer of the torque transmitter should use the materials specified in Appendix A of YY/T 1486-2016.
The hardness of steel rods is tested by the method specified in GB/T 4340.1, and the Vickers hardness should not be less than 500HV5.
7 Performance evaluation
Chapter 8 of YY/T 1486-2016 is applicable.
8 Production
Chapter 9 of YY/T 1486-2016 applies.
The torque transmitter shall be produced in accordance with the requirements of Chapter 6 and Chapter 7 of this standard.
The test is carried out in accordance with the method specified in 11.1.
9 Repeated treatment tolerance
Chapter 10 of YY/T 1486-2016 applies.
All devices that are expected to be used multiple times should withstand a repeated treatment cycle of 100 times, and according to the manufacturer's instructions, there should be no performance
Signs of decline or corrosion.
The repeated treatment cycle should include the recommended cleaning, disinfection and sterilization methods.
10 Information provided by the manufacturer
Chapter 11 of YY/T 1486-2016 applies.
The shape and size of the part of the implant or connecting part connected to the torque transmitter should at least be included in the label information.
Where appropriate, the manufacturer's name or registered trademark should be marked on the device and related auxiliary devices.
11 Test method
11.1 Technical documents for inspection products
Check the product technical documents to determine whether it meets the requirements.
11.2 Dimensions
Through visual inspection and the use of suitable measuring instruments for measurement, to determine whether it meets the requirements of all dimensions described by the manufacturer.
11.3 Locked-rotor torque
11.3.1 Instruments
The instrument requirements are as follows.
a) The clamping system complies with YY/T 0967.1-2015.
b) Torque meter or dynamometer can measure torque in Newton meters with an accuracy of ±10%.
11.3.2 Procedure
The torque transmitter is inserted into the clamping system and fixed, the torque measuring device is connected to the outer end of the torque transmitter, and the torque meter is slowly rotated until it reaches the
When the maximum allowable torque is reached, no deformation should be observed.
Share