1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1709-2020 English PDF (YY/T1709-2020)
YY/T 1709-2020 English PDF (YY/T1709-2020)
Regular price
$325.00 USD
Regular price
Sale price
$325.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1709-2020 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1709-2020
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1709-2020: Evaluation of measurement uncertainty of calibrators for in vitro diagnostic kits
YY/T 1709-2020
Evaluation of measurement uncertainty of calibrators for in vitro diagnostic kits
ICS 11.100
C44
People's Republic of China Pharmaceutical Industry Standard
Evaluation of Measurement Uncertainty of Calibrators for In Vitro Diagnostic Reagents
2020-06-30 released
2021-12-01 implementation
Issued by the State Drug Administration
Evaluation of Uncertainty in Measurement of Calibrators for In Vitro Diagnostic Reagents
1 Scope
This standard specifies the evaluation method for the measurement uncertainty of the calibrator used in in vitro diagnostic reagents.
This standard is applicable to the evaluation of uncertainty in measurement of product calibrators for in vitro diagnostic quantitative reagents.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 21415 Metrology traceability of measurement calibrators and control substance assignments in biological samples of in vitro diagnostic medical devices
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Product calibrator (product)
Calibrator (product)
A calibrator intended to be used in the manufacturer's final product.
Note. The product calibrator here includes the calibrator used by the manufacturer to calibrate the final product. The calibration information of the calibrator will be transmitted through electronic carriers.
Handed to the measurement of clinical samples.
3.2
Working calibrator (product)
Main calibrator (product)
The measurement standard used for calibration of the manufacturer's permanent measurement program.
3.3
Metrological traceability
The characteristic of linking the measurement results with the reference through the uninterrupted calibration chain specified in the document, and each calibration will introduce measurement uncertainty
Fixed degree.
[GB/T 29791.1-2013, definition 3.48]
3.4
Measurement uncertainty
uncertainty
According to the information used, it characterizes the non-negative parameters that give the measured value dispersion.
[GB/T 29791.1-2013, definition A.3.35]
3.5
Target uncertainty
Maximum allowable measurement uncertainty
The upper limit of the uncertainty of measurement determined based on the intended use of the measurement result.
3.6
Measurement precision
Under specified conditions, the degree of agreement between mutually independent measurement results.
Note 1.Measurement precision cannot give the measured value, and can only be described as "sufficient" or "insufficient" for the specified purpose.
Note 2.The degree of precision is usually expressed by measurement imprecision statistics that are opposite to precision, such as standard deviation and coefficient of variation.
Note 3.The "precision" of a given measurement procedure can be classified according to specific precision conditions. "Repeatability" is related to basically unchanged conditions, often called
"Intra-sequence precision" and "Intra-assay precision". "Reproducibility" is related to changing conditions, such as. time, different laboratories, different operators and different measurements
The precision of the system (including different calibrations and reagent lot numbers).
[GB/T 21415-2008, definition 3.23]
3.7
Standard measurement uncertainty
Standard uncertainty
The measurement uncertainty expressed in standard deviation.
[JJF1001-2011, definition 5.19]
3.8
Relative standard measurement uncertainty
Relative standard uncertainty
The standard uncertainty is divided by the absolute value of the measured value.
[JJF1001-2011, definition 5.23]
3.9
Synthetic standard measurement uncertainty
Synthetic standard uncertainty
The standard measurement uncertainty of the output obtained by the standard measurement uncertainty of each input in a measurement model.
[JJF1001-2011, definition 5.22]
3.10
Extended measurement uncertainty
Extended uncertainty
The product of the composite standard uncertainty and a numerical factor greater than 1.
Note. "Factor" refers to the inclusion factor.
[JJF1001-2011, definition 5.27]
3.11
Inclusion probability
The probability that a set of measured values is contained within the specified containment interval.
[JJF1001-2011, definition 5.29]
3.12
Inclusion factor
In order to obtain the expanded uncertainty, the composite standard uncertainty is multiplied by a number greater than 1.
[JJF1001-2011, definition 5.30]
They should be modified or supplemented, but they should be combined with specific products to comprehensively evaluate each uncertainty element, and adopt a standardized evaluation process and scientific statistical methods.
Method, keep data records and calculation process to ensure that the calibrator assignment and its uncertainty meet clinical needs.
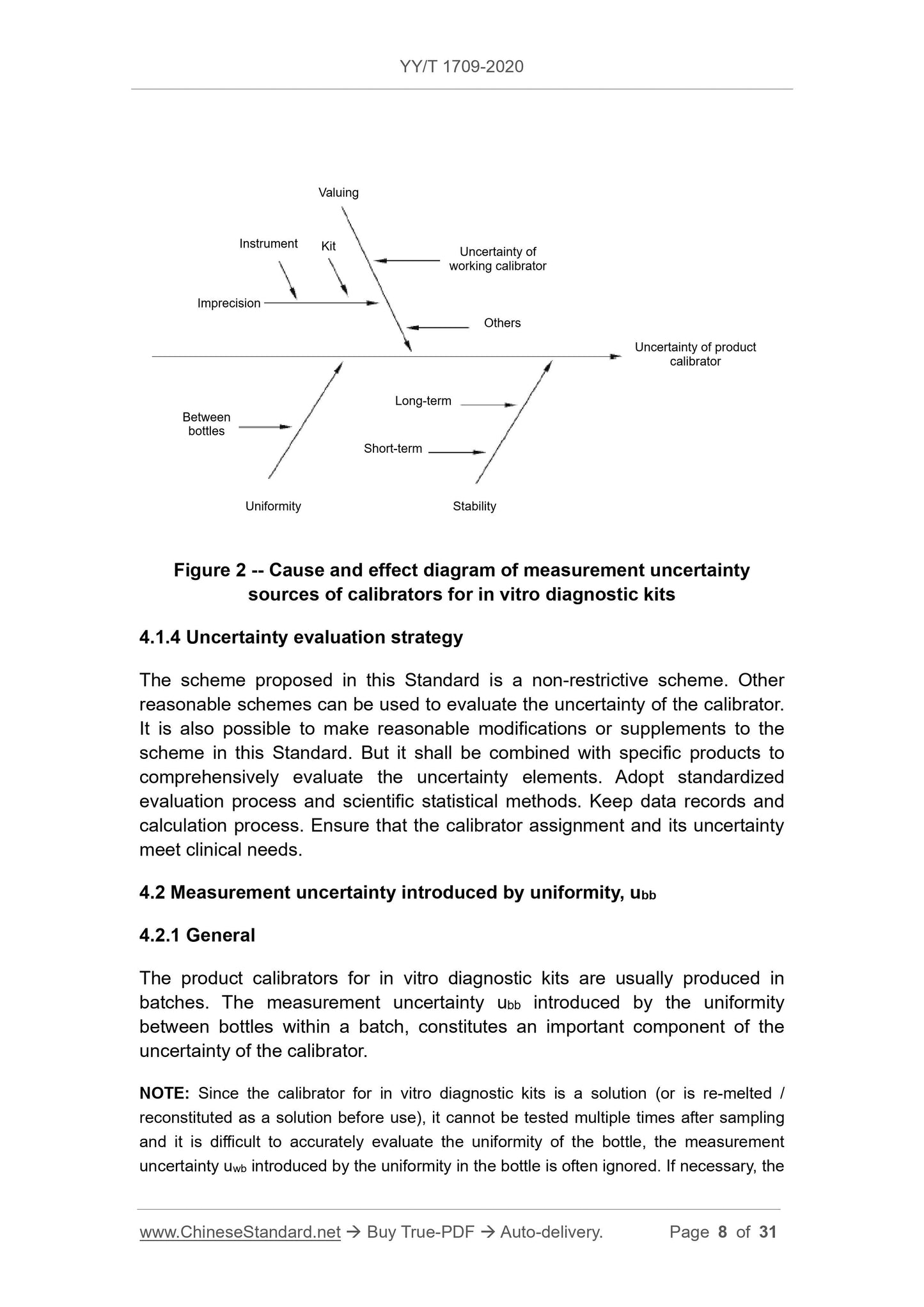
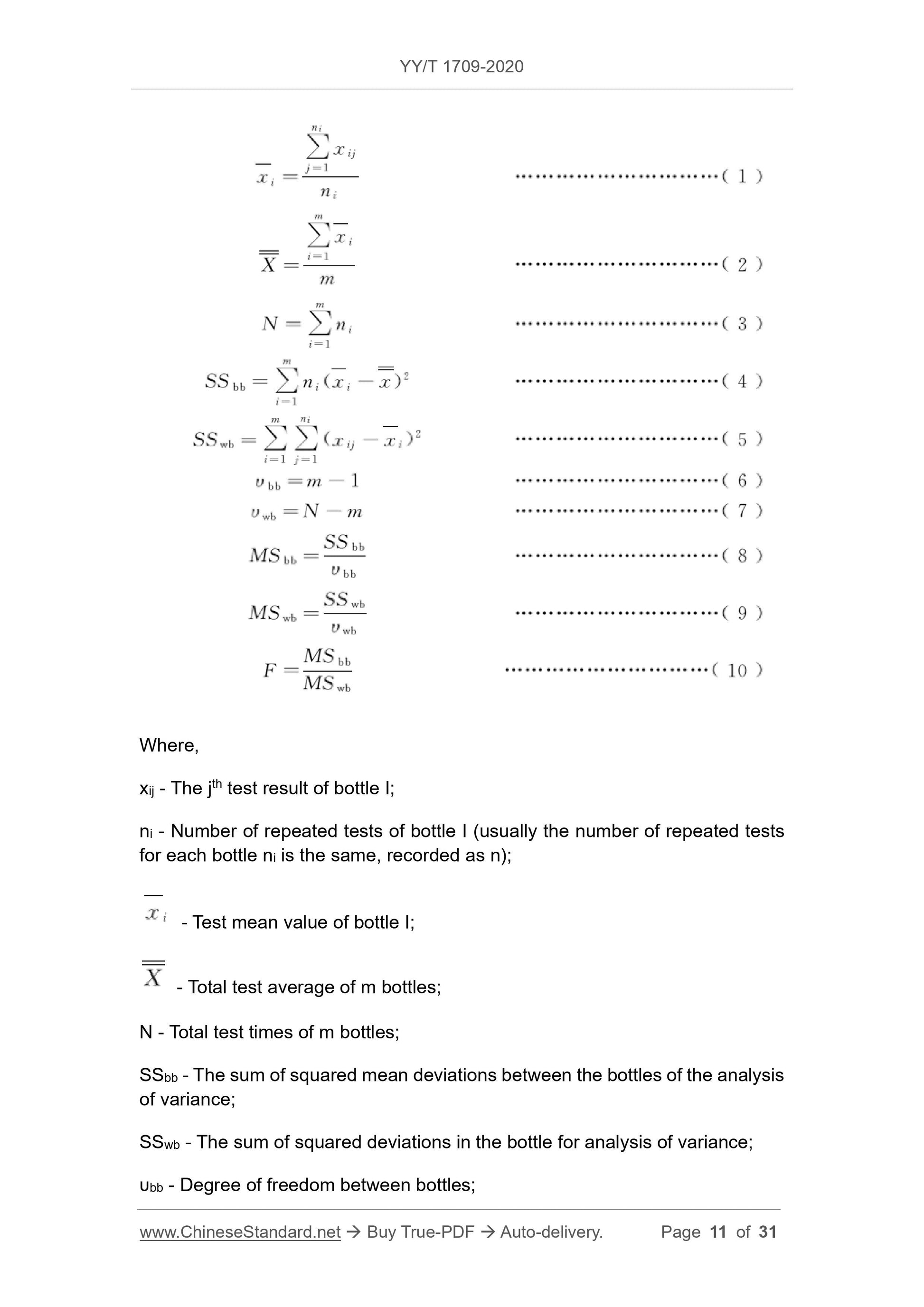
4.2 Measurement uncertainty introduced by uniformity ubb
4.2.1 General
Product calibrators for in vitro diagnostic reagents are usually produced in batches, and the measurement uncertainty ubb introduced by the uniformity between bottles within a batch constitutes an important component of the uncertainty of the calibrators.
Note. Since the calibrator for in-vitro diagnostic reagents is a solution (or remelted/reconstituted as a solution before use), and cannot be tested multiple times after sampling, it is difficult to even
The uniformity is accurately evaluated, so the measurement uncertainty uwb introduced by the uniformity in the bottle is often ignored. If necessary, the repeatability standard of the test method can be used.
Estimate the quasi-bias sr.
If there are more than 2 concentrations of product calibrators, the uniformity test of each concentration of calibrators is required (except for zero-concentration calibrators).
If the product calibrator contains multiple test items, the uniformity of each test item needs to be evaluated separately, unless there is a clear distribution relationship between the two items.
4.2.2 Test plan
4.2.2.1 Test method
The test method should meet the following requirements.
a) The manufacturer's permanent measurement procedures or other measurement procedures that set the value of the product calibrator can be used, and the test can be completed under repeatable conditions.
b) The reportable concentration range should cover the expected concentration of the product calibrator. If the expected concentration of the product calibrator is higher than the test system
Concentration range, the product calibrator can be accurately diluted by weighing under strictly specified test conditions and guaranteed
This dilution will not change the interchangeability of the calibrators and thus affect the uniformity test results.
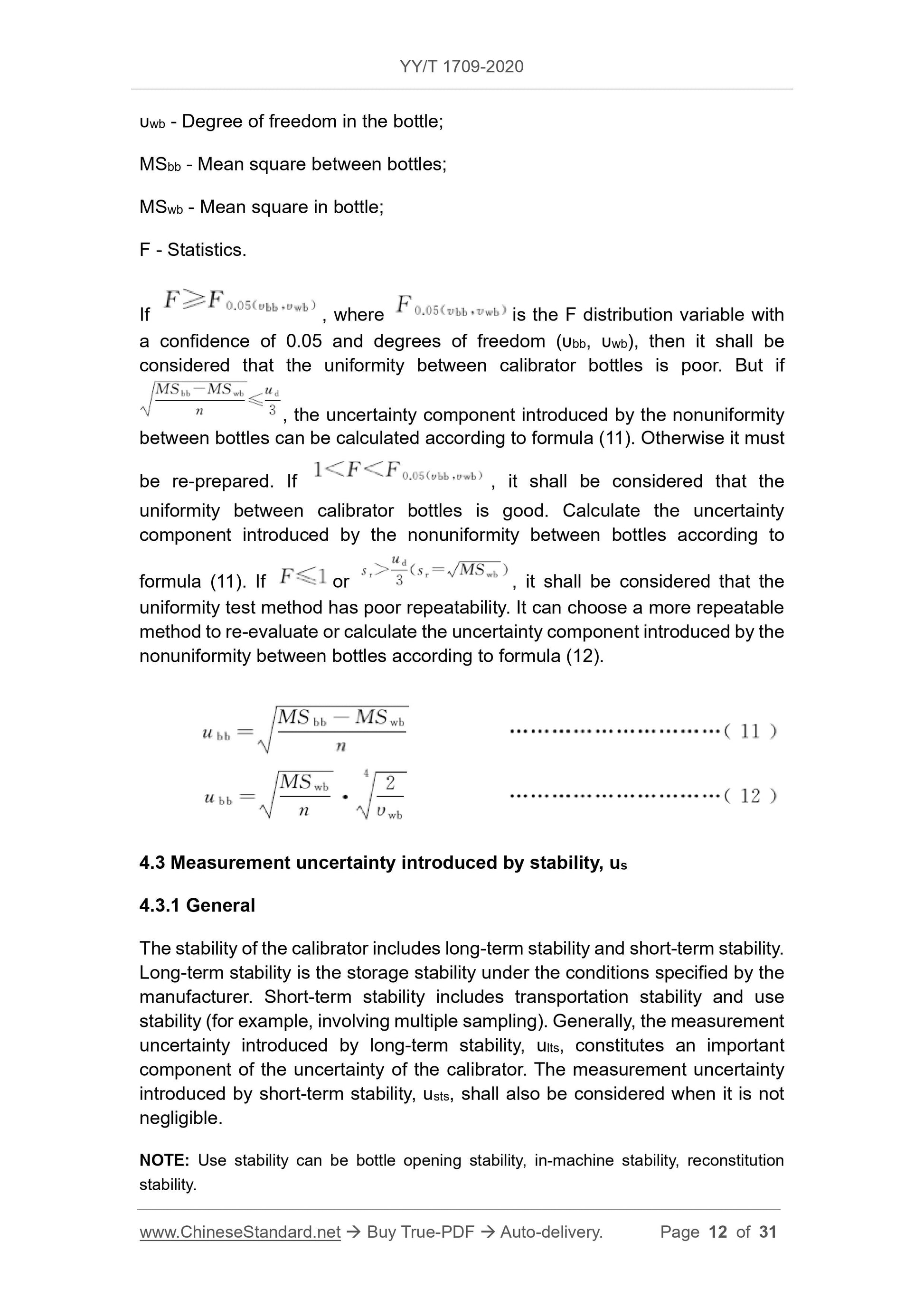
c) The precision of the method should be able to reflect the difference between bottles, and should not be inferior to the precision of the fixed value method. Under ideal conditions, sr≤ud, where sr is
The repeatability standard deviation of the test method, ud is the target standard uncertainty of the calibrator.
d) The minimum sampling amount that can ensure uniformity should be specified, and it is recommended that it should not be higher than the sampling amount when calibrating the kit.
Note. If the calibrator is diluted and then used to calibrate the matching kit, the minimum sampling volume for homogeneity testing shall not be higher than the minimum sampling volume (ladder
For degree dilution, take the minimum sampling amount of the first dilution operation).
4.2.2.2 Sampling
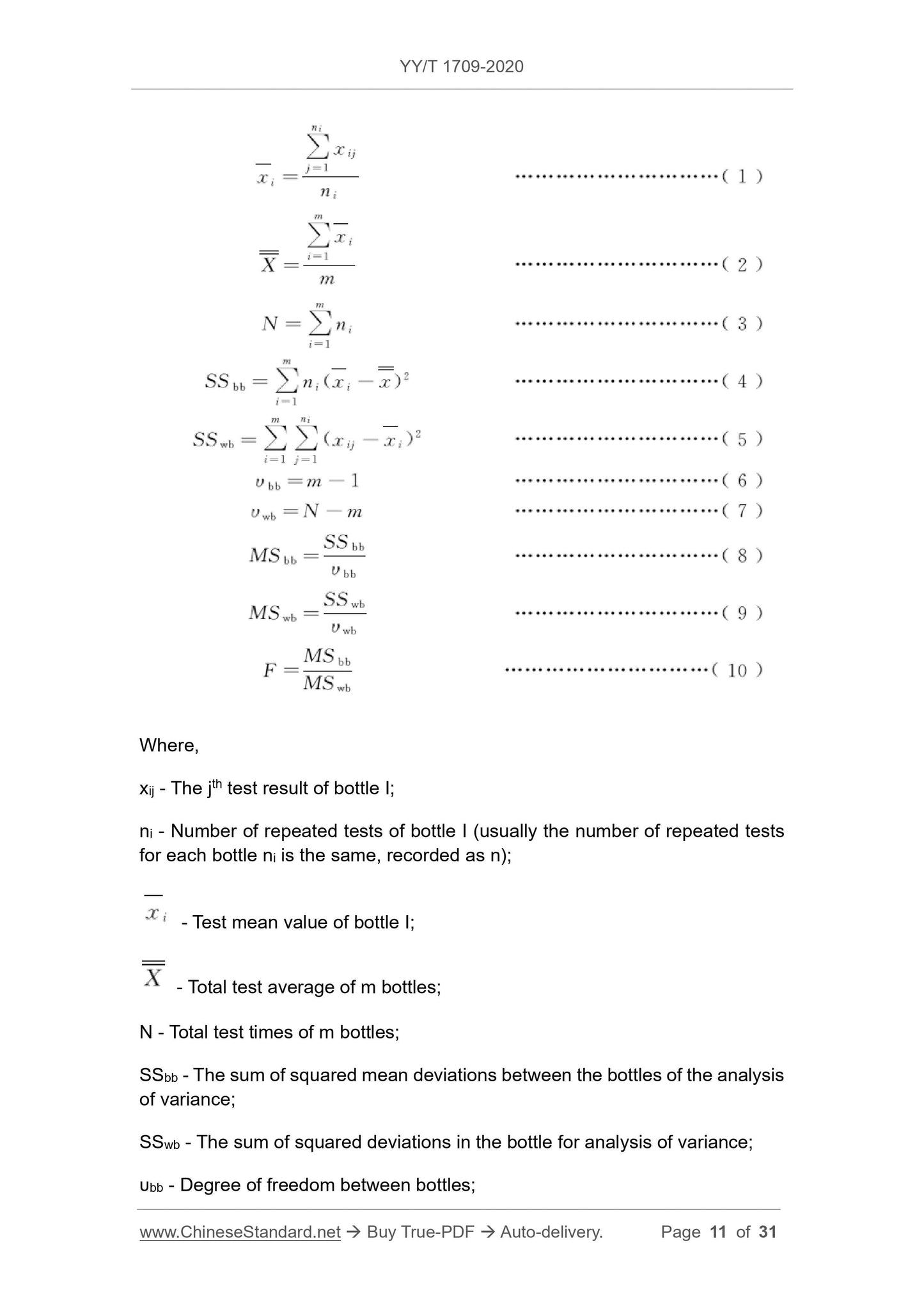
The calibrator of the smallest packaging unit is selected for uniformity inspection according to the random stratification method, and the samples are numbered sequentially, such as 1, 2, 15.
Considering that the calibrator for in vitro diagnostic reagents is a solution (or remelted/reconstituted into a solution before use), the uniformity is good, and MAX (10, 3Nprod) is drawn
Packages (Nprod is batch production). For test items with poor uniformity or uniformity test methods with poor repeatability,
The sample volume can be increased appropriately. If Nprod≤100, extract MAX(3,10%Nprod) packages, and appropriately increase the number of repeated determinations for each package unit.
4.2.2.3 Test
Each packaging unit shall be measured at least 3 times. Considering the fluctuation of the measurement system over time and other factors, it is necessary to change between 3 measurements.
Reverse the order of the samples, such as 1-3-5-7-9-11-13-15-2-4-6-8-10-12-14-15-14-13-12-11-10-9- 8-7-6-5-4-3-2-1-2-4-6-8-
10-12-14-1-3-5-7-9-11-13-15.
It is recommended that the uncertainty of the product calibrator be reported in the form of standard uncertainty "(digital value assigned ±uc) unit". If the extension is not
The form of certainty "(assignment ± U numerical value) unit" report must clearly include the value of factor k.
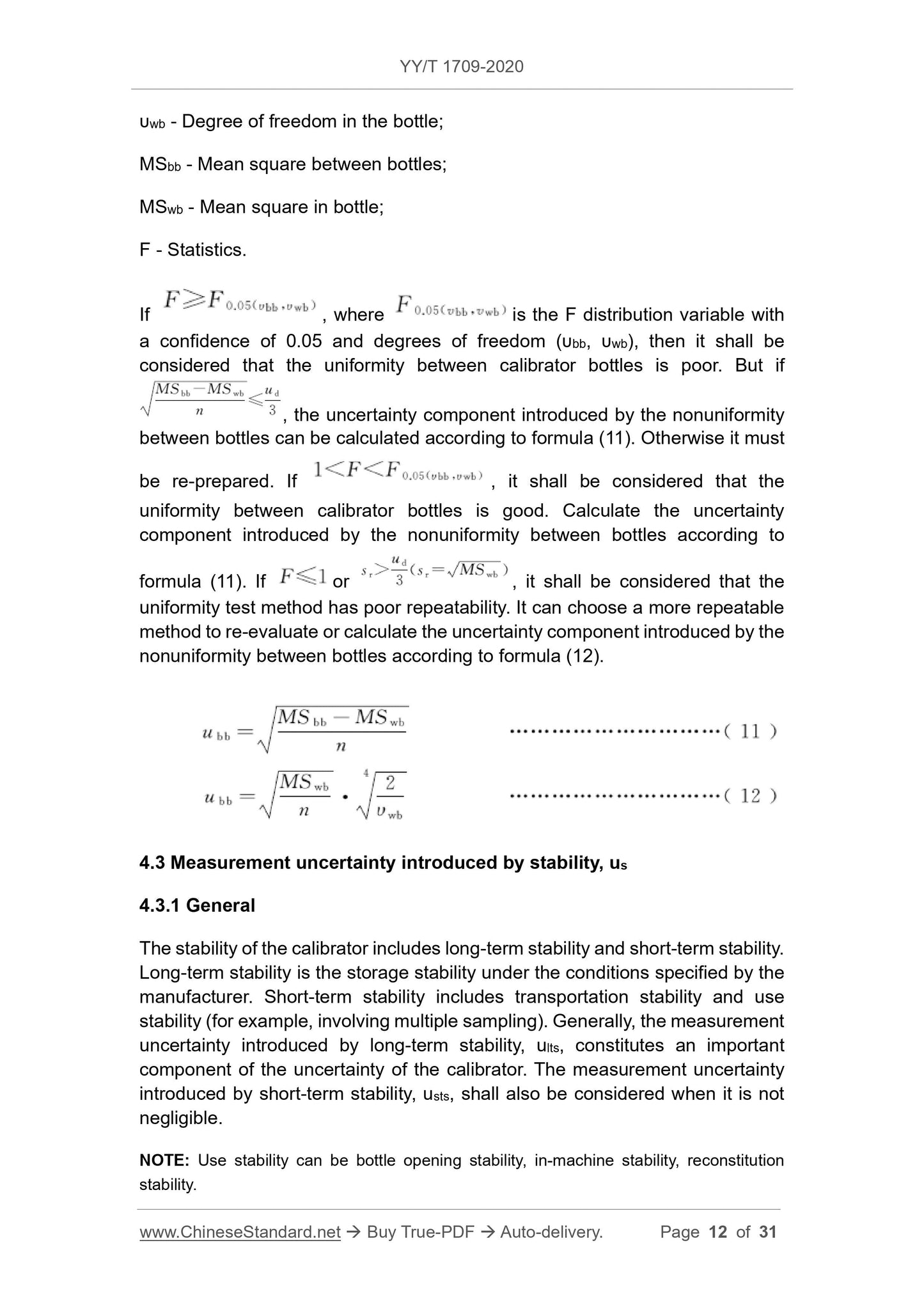
If the measurement uncertainty of the calibrator is less than the preset target uncertainty, the traceability of the fixed value of the calibrator can be confirmed. Otherwise, it should be divided
Analyze and find the reasons, improve the preparation process or test system, change the traceability chain if necessary, and re-apply the calibrator assignment and uncertainty evaluation.
5 Uncertainty of re-batch calibrator
Usually the calibrator needs to re-evaluate the uncertainty when changing batches.
If the historical batch data of uncertainty component or synthetic uncertainty is used, the following conditions must be met.
a) The characteristic value of the calibrator changes within ±10%;
b) The source of key raw materials and article numbers have not changed, and the preparation process has not changed;
c) The calibrator assignment system (including traceability chain, working calibrator, equipment and operating procedures, etc.) has not changed;
d) There have been 3 consecutive batches or more of calibrator uncertainty evaluation data, showing that the variation of the synthetic standard uncertainty is within the allowable range specified by the manufacturer.
If both a) and b) are satisfied, the stability uncertainty component of the historical batch can be quoted, but the stability should still be monitored in real time. Manufacturer can
Accumulation of multiple batches of stability data is used as the basis for assessing the uncertainty component introduced in stability.
If a) ~ d) are satisfied at the same time, the maximum uncertainty of the historical batch can be selected as the uncertainty of the future batch of calibrators. Manufacturer still
The validity of the uncertainty should be verified regularly to ensure that the requirements of the target uncertainty are met, and changes in technical capabilities should be reflected in time.
Get Quotation: Click YY/T 1709-2020 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1709-2020
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1709-2020: Evaluation of measurement uncertainty of calibrators for in vitro diagnostic kits
YY/T 1709-2020
Evaluation of measurement uncertainty of calibrators for in vitro diagnostic kits
ICS 11.100
C44
People's Republic of China Pharmaceutical Industry Standard
Evaluation of Measurement Uncertainty of Calibrators for In Vitro Diagnostic Reagents
2020-06-30 released
2021-12-01 implementation
Issued by the State Drug Administration
Evaluation of Uncertainty in Measurement of Calibrators for In Vitro Diagnostic Reagents
1 Scope
This standard specifies the evaluation method for the measurement uncertainty of the calibrator used in in vitro diagnostic reagents.
This standard is applicable to the evaluation of uncertainty in measurement of product calibrators for in vitro diagnostic quantitative reagents.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 21415 Metrology traceability of measurement calibrators and control substance assignments in biological samples of in vitro diagnostic medical devices
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Product calibrator (product)
Calibrator (product)
A calibrator intended to be used in the manufacturer's final product.
Note. The product calibrator here includes the calibrator used by the manufacturer to calibrate the final product. The calibration information of the calibrator will be transmitted through electronic carriers.
Handed to the measurement of clinical samples.
3.2
Working calibrator (product)
Main calibrator (product)
The measurement standard used for calibration of the manufacturer's permanent measurement program.
3.3
Metrological traceability
The characteristic of linking the measurement results with the reference through the uninterrupted calibration chain specified in the document, and each calibration will introduce measurement uncertainty
Fixed degree.
[GB/T 29791.1-2013, definition 3.48]
3.4
Measurement uncertainty
uncertainty
According to the information used, it characterizes the non-negative parameters that give the measured value dispersion.
[GB/T 29791.1-2013, definition A.3.35]
3.5
Target uncertainty
Maximum allowable measurement uncertainty
The upper limit of the uncertainty of measurement determined based on the intended use of the measurement result.
3.6
Measurement precision
Under specified conditions, the degree of agreement between mutually independent measurement results.
Note 1.Measurement precision cannot give the measured value, and can only be described as "sufficient" or "insufficient" for the specified purpose.
Note 2.The degree of precision is usually expressed by measurement imprecision statistics that are opposite to precision, such as standard deviation and coefficient of variation.
Note 3.The "precision" of a given measurement procedure can be classified according to specific precision conditions. "Repeatability" is related to basically unchanged conditions, often called
"Intra-sequence precision" and "Intra-assay precision". "Reproducibility" is related to changing conditions, such as. time, different laboratories, different operators and different measurements
The precision of the system (including different calibrations and reagent lot numbers).
[GB/T 21415-2008, definition 3.23]
3.7
Standard measurement uncertainty
Standard uncertainty
The measurement uncertainty expressed in standard deviation.
[JJF1001-2011, definition 5.19]
3.8
Relative standard measurement uncertainty
Relative standard uncertainty
The standard uncertainty is divided by the absolute value of the measured value.
[JJF1001-2011, definition 5.23]
3.9
Synthetic standard measurement uncertainty
Synthetic standard uncertainty
The standard measurement uncertainty of the output obtained by the standard measurement uncertainty of each input in a measurement model.
[JJF1001-2011, definition 5.22]
3.10
Extended measurement uncertainty
Extended uncertainty
The product of the composite standard uncertainty and a numerical factor greater than 1.
Note. "Factor" refers to the inclusion factor.
[JJF1001-2011, definition 5.27]
3.11
Inclusion probability
The probability that a set of measured values is contained within the specified containment interval.
[JJF1001-2011, definition 5.29]
3.12
Inclusion factor
In order to obtain the expanded uncertainty, the composite standard uncertainty is multiplied by a number greater than 1.
[JJF1001-2011, definition 5.30]
They should be modified or supplemented, but they should be combined with specific products to comprehensively evaluate each uncertainty element, and adopt a standardized evaluation process and scientific statistical methods.
Method, keep data records and calculation process to ensure that the calibrator assignment and its uncertainty meet clinical needs.
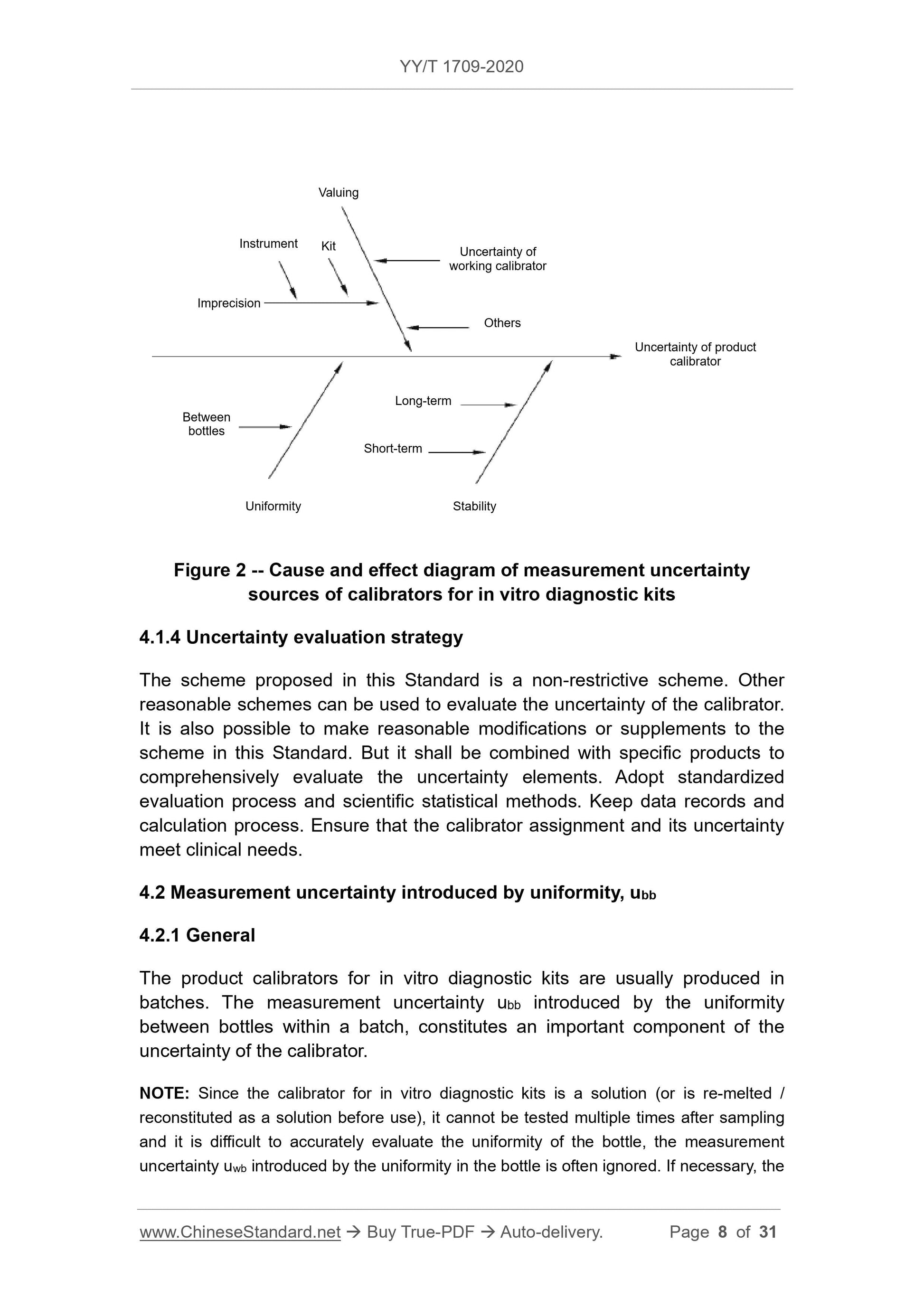
4.2 Measurement uncertainty introduced by uniformity ubb
4.2.1 General
Product calibrators for in vitro diagnostic reagents are usually produced in batches, and the measurement uncertainty ubb introduced by the uniformity between bottles within a batch constitutes an important component of the uncertainty of the calibrators.
Note. Since the calibrator for in-vitro diagnostic reagents is a solution (or remelted/reconstituted as a solution before use), and cannot be tested multiple times after sampling, it is difficult to even
The uniformity is accurately evaluated, so the measurement uncertainty uwb introduced by the uniformity in the bottle is often ignored. If necessary, the repeatability standard of the test method can be used.
Estimate the quasi-bias sr.
If there are more than 2 concentrations of product calibrators, the uniformity test of each concentration of calibrators is required (except for zero-concentration calibrators).
If the product calibrator contains multiple test items, the uniformity of each test item needs to be evaluated separately, unless there is a clear distribution relationship between the two items.
4.2.2 Test plan
4.2.2.1 Test method
The test method should meet the following requirements.
a) The manufacturer's permanent measurement procedures or other measurement procedures that set the value of the product calibrator can be used, and the test can be completed under repeatable conditions.
b) The reportable concentration range should cover the expected concentration of the product calibrator. If the expected concentration of the product calibrator is higher than the test system
Concentration range, the product calibrator can be accurately diluted by weighing under strictly specified test conditions and guaranteed
This dilution will not change the interchangeability of the calibrators and thus affect the uniformity test results.
c) The precision of the method should be able to reflect the difference between bottles, and should not be inferior to the precision of the fixed value method. Under ideal conditions, sr≤ud, where sr is
The repeatability standard deviation of the test method, ud is the target standard uncertainty of the calibrator.
d) The minimum sampling amount that can ensure uniformity should be specified, and it is recommended that it should not be higher than the sampling amount when calibrating the kit.
Note. If the calibrator is diluted and then used to calibrate the matching kit, the minimum sampling volume for homogeneity testing shall not be higher than the minimum sampling volume (ladder
For degree dilution, take the minimum sampling amount of the first dilution operation).
4.2.2.2 Sampling
The calibrator of the smallest packaging unit is selected for uniformity inspection according to the random stratification method, and the samples are numbered sequentially, such as 1, 2, 15.
Considering that the calibrator for in vitro diagnostic reagents is a solution (or remelted/reconstituted into a solution before use), the uniformity is good, and MAX (10, 3Nprod) is drawn
Packages (Nprod is batch production). For test items with poor uniformity or uniformity test methods with poor repeatability,
The sample volume can be increased appropriately. If Nprod≤100, extract MAX(3,10%Nprod) packages, and appropriately increase the number of repeated determinations for each package unit.
4.2.2.3 Test
Each packaging unit shall be measured at least 3 times. Considering the fluctuation of the measurement system over time and other factors, it is necessary to change between 3 measurements.
Reverse the order of the samples, such as 1-3-5-7-9-11-13-15-2-4-6-8-10-12-14-15-14-13-12-11-10-9- 8-7-6-5-4-3-2-1-2-4-6-8-
10-12-14-1-3-5-7-9-11-13-15.
It is recommended that the uncertainty of the product calibrator be reported in the form of standard uncertainty "(digital value assigned ±uc) unit". If the extension is not
The form of certainty "(assignment ± U numerical value) unit" report must clearly include the value of factor k.
If the measurement uncertainty of the calibrator is less than the preset target uncertainty, the traceability of the fixed value of the calibrator can be confirmed. Otherwise, it should be divided
Analyze and find the reasons, improve the preparation process or test system, change the traceability chain if necessary, and re-apply the calibrator assignment and uncertainty evaluation.
5 Uncertainty of re-batch calibrator
Usually the calibrator needs to re-evaluate the uncertainty when changing batches.
If the historical batch data of uncertainty component or synthetic uncertainty is used, the following conditions must be met.
a) The characteristic value of the calibrator changes within ±10%;
b) The source of key raw materials and article numbers have not changed, and the preparation process has not changed;
c) The calibrator assignment system (including traceability chain, working calibrator, equipment and operating procedures, etc.) has not changed;
d) There have been 3 consecutive batches or more of calibrator uncertainty evaluation data, showing that the variation of the synthetic standard uncertainty is within the allowable range specified by the manufacturer.
If both a) and b) are satisfied, the stability uncertainty component of the historical batch can be quoted, but the stability should still be monitored in real time. Manufacturer can
Accumulation of multiple batches of stability data is used as the basis for assessing the uncertainty component introduced in stability.
If a) ~ d) are satisfied at the same time, the maximum uncertainty of the historical batch can be selected as the uncertainty of the future batch of calibrators. Manufacturer still
The validity of the uncertainty should be verified regularly to ensure that the requirements of the target uncertainty are met, and changes in technical capabilities should be reflected in time.
Share