1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1710-2020 English PDF (YYT1710-2020)
YY/T 1710-2020 English PDF (YYT1710-2020)
Regular price
$245.00 USD
Regular price
Sale price
$245.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1710-2020
Historical versions: YY/T 1710-2020
Preview True-PDF (Reload/Scroll if blank)
YY/T 1710-2020: Disposable abdominal trocars
YY/T 1710-2020
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Disposable Abdominal Trocars
ISSUED ON: FEBRUARY 26, 2020
IMPLEMENTED ON: MARCH 1, 2021
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
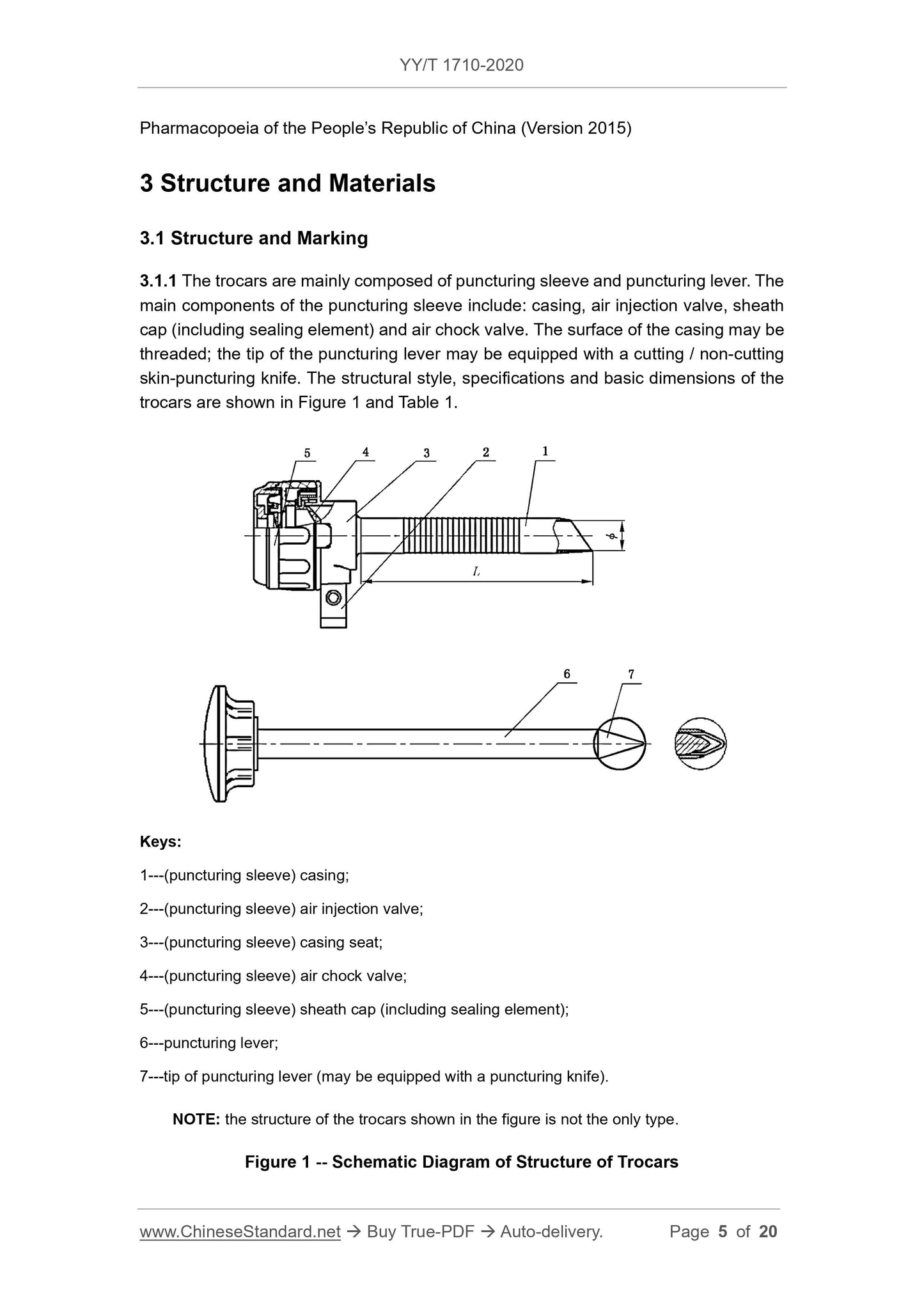
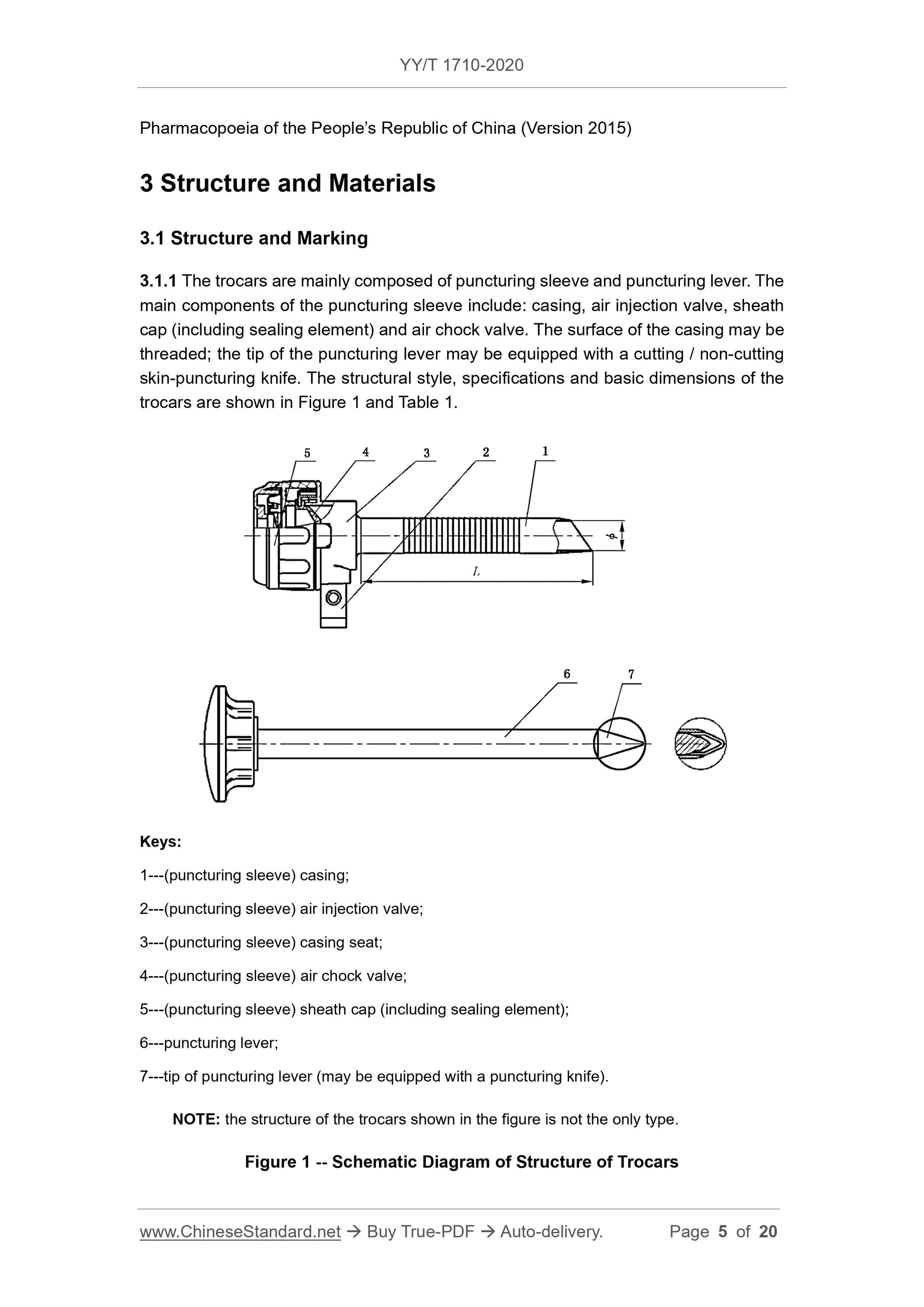
3 Structure and Materials ... 5
4 Requirements... 7
5 Test Methods ... 10
6 Type Inspection ... 13
7 Labeling and Instruction Manual ... 13
8 Packaging ... 14
Appendix A (normative) Test Method for Air Chocking and Sealing Performance
... 15
Appendix B (informative) Evaluation and Test Method for Puncturing
Performance ... 17
Appendix C (informative) Evaluation and Test Method for Plugging / Unplugging
Performance ... 19
Disposable Abdominal Trocars
1 Scope
This Standard specifies the structure, materials, requirements, test methods, type
inspection, labeling, instruction manual and packaging of disposable abdominal trocars.
This Standard is applicable to disposable abdominal trocars (hereinafter referred to as
trocars) that puncture human abdominal wall tissues during laparoscopic surgery to
establish artificial pneumoperitoneum and operate surgical instrument channel.
2 Normative References
The following documents are indispensable to the application of this document. In
terms of references with a specified date, only versions with a specified date are
applicable to this document. In terms of references without a specified date, the latest
version (including all the modifications) is applicable to this document.
GB/T 1220 Stainless Steel Bars
GB/T 1962.2 Conical Fittings with a 6% (Luer) Taper for Syringes, Needles and Certain
Other Medical Equipment - Part 2: Lock Fittings
GB/T 3280 Cold Rolled Stainless Steel Plate, Sheet and Strip
GB/T 4340.1 Metallic Materials - Vickers Hardness Test - Part 1: Test Method
GB/T 6682-2008 Water for Analytical Laboratory Use - Specification and Test Methods
GB/T 12672 Acrylonitrile-butadiene-styrene (ABS) Resin
GB/T 14233.1-2008 Test Methods for Infusion, Transfusion, Injection Equipment for
Medical Use - Part 1: Chemical Analysis Methods
GB/T 16886 (all parts) Biological Evaluation of Medical Devices
YY/T 0149-2006 Medical Instruments of Stainless Steel - Test Methods of Corrosion
Resistance
YY/T 0466.1 Medical Devices - Symbols to be Used with Medical Device Labels,
Labelling and Information to be Supplied - Part 1: General Requirements
YY/T 0806 Polycarbonate Material for Manufacture of Infusion, Transfusion and
Injection Equipment for Medical Use and Other Medical Devices
4 Requirements
4.1 Appearance
4.1.1 The outer surface of the trocars shall be smooth and clean. There shall be no
defects, such as: burrs, bubbles, impurities, cracks and sintered substances, etc.
4.1.2 The surface of the trocars shall not have visible accumulation of lubricant.
4.1.3 The specification marking on the body of the trocars shall be clearly visible.
4.1.4 If there is a puncturing knife at the tip of the puncturing lever, then, the puncturing
knife shall be flat, and there shall be no rust, sharp edges, burrs or obvious pitting. The
cutting edge of the puncturing knife shall be free of nicks, white edges, wire edges and
cracks, etc.
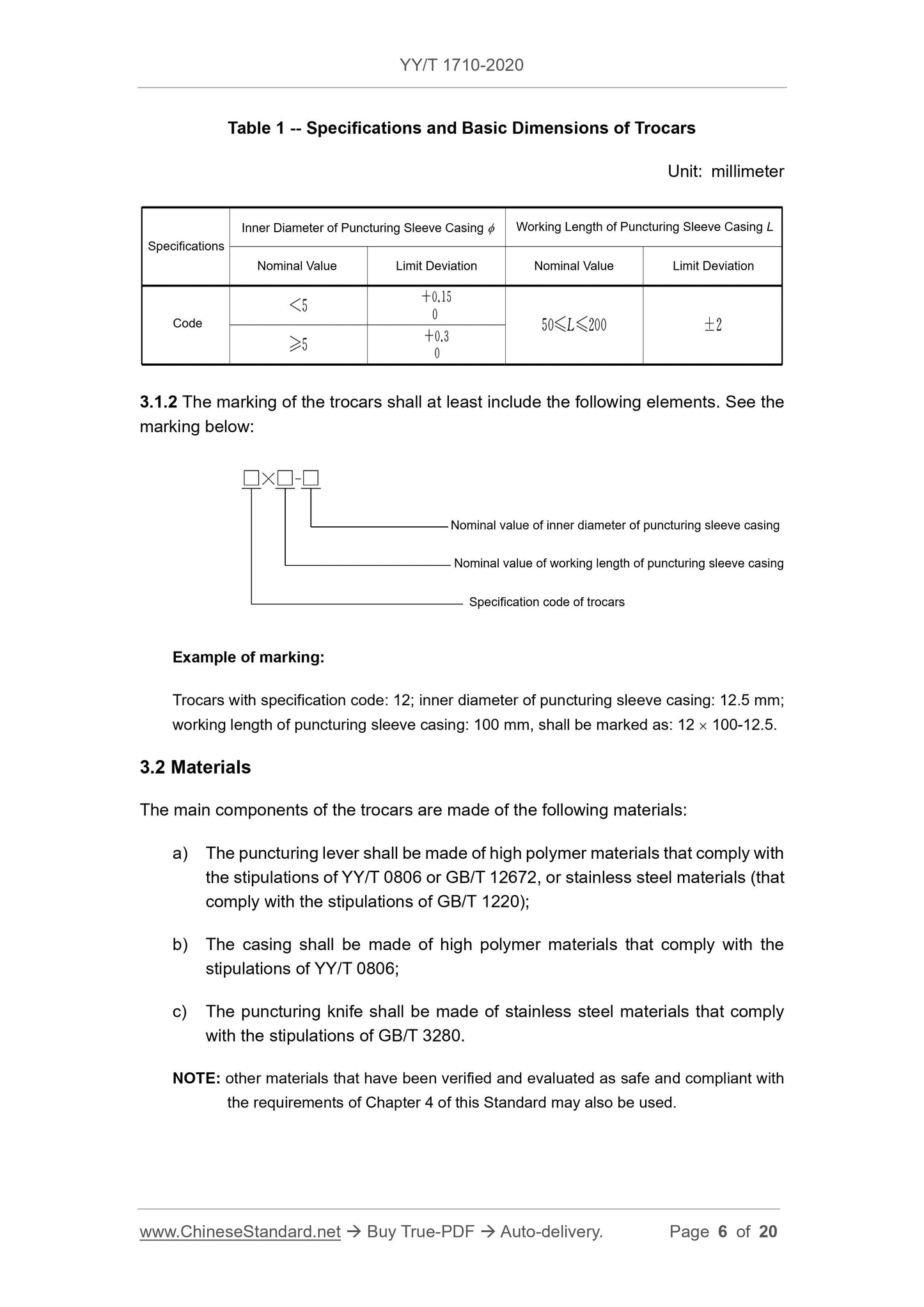
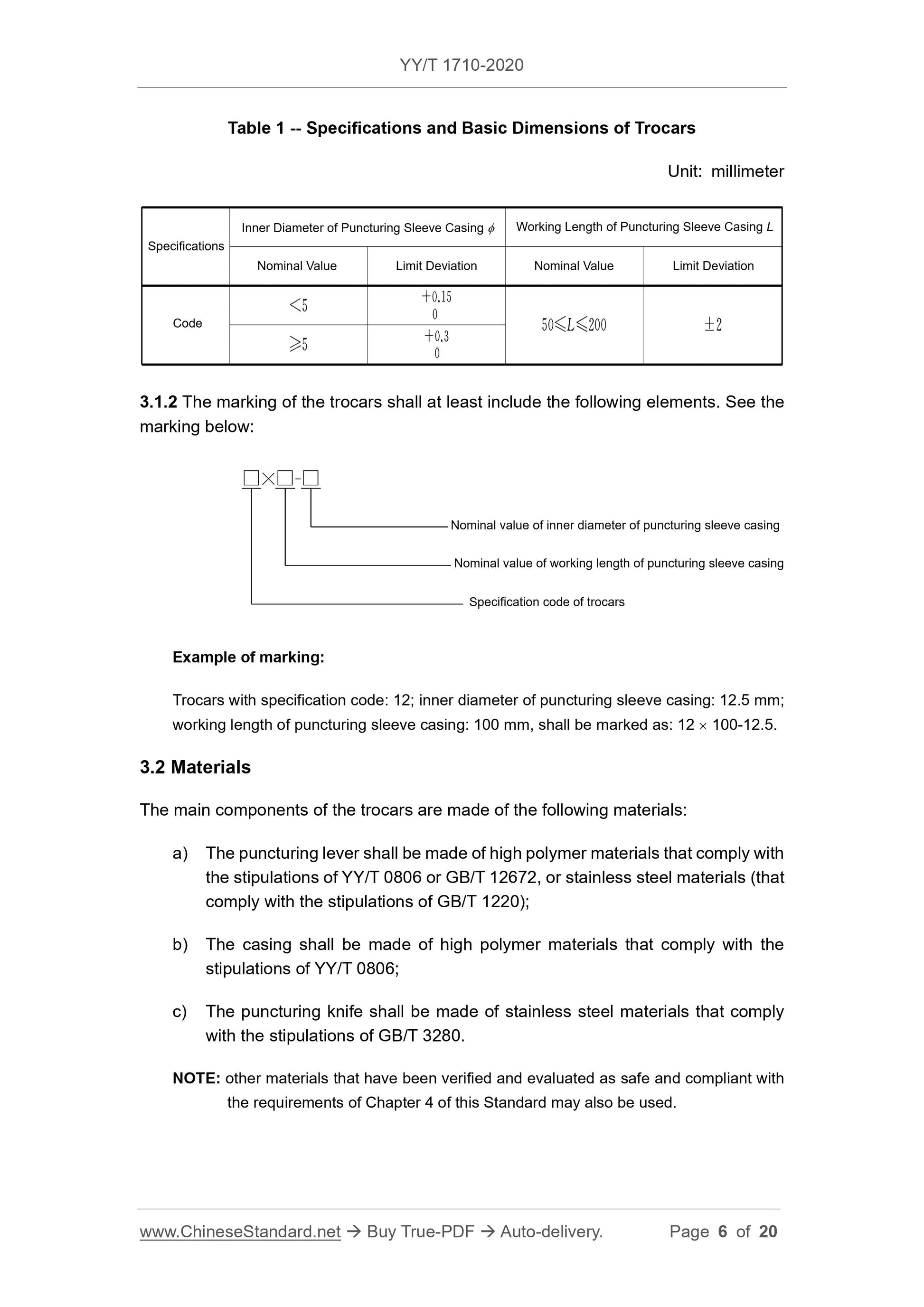
4.2 Dimensions
The inner diameter and working length L of the puncturing sleeve casing of the
trocars shall comply with the stipulations of Table 1.
4.3 Surface Roughness
If there is a puncturing knife at the tip of the puncturing lever, then, the surface
roughness of the cutting edge surface of the puncturing knife Ra ≤ 0.8 μm.
4.4 Hardness
If there is a skin-puncturing knife at the tip of the puncturing lever, then, the puncturing
knife shall receive thermal treatment; its hardness shall be not less than 650HV10.
4.5 Flexibility
4.5.1 The air injection valve of the trocars shall be able to be flexibly opened and closed;
there shall be no obstruction or jamming.
4.5.2 If the sheath cap is detachable, then, its assembly and disassembly shall be
flexible and convenient; there shall be no obstruction or jamming.
4.5.3 If there is a puncturing knife at the tip of the puncturing lever, then, the puncturing
knife shall be able to be flexibly retracted; there shall be no obstruction or jamming.
4.6 Coordination Performance
4.6.1 The puncturing sleeve and the puncturing lever shall properly coordinate with
each other. There shall be no jamming when plugging or unplugging.
shall be not greater than 10 μg/g.
4.14 Dissolved Precipitates of Polymer Materials of Parts in Contact with
Patient
4.14.1 Appearance (turbidity and color)
The dissolution liquid shall be colorless and transparent, and there shall be no visible
foreign objects.
4.14.2 pH
The pH difference between the dissolution liquid and the blank control solution of the
same batch shall be ≤ 2.0.
4.14.3 Heavy metals
The total content of heavy metals that can be dissolved in the dissolution liquid shall
be ≤ 5 μg/mL.
4.14.4 Reducing substance
Compare the dissolution liquid with the same batch of blank control solution of an equal
volume; the difference in the amount of consumed potassium permanganate solution
[c (1/5KMnO4) = 0.01 mol/L] shall be ≤ 2.0 mL.
4.14.5 Evaporation residue
The total dry residue of the dissolution liquid shall be ≤ 2.0 mg.
4.15 Package Marking and Instruction Manual
4.15.1 The single package of the trocars shall have a marking that complies with the
stipulations of 3.1.2.
4.15.2 The instruction manual of the trocars shall include the specifications of devices
that can be used together with the trocars.
4.15.3 If the puncturing knife has the function of skin-puncturing, then, it shall be clearly
described in the instruction manual.
4.16 Biological Evaluation
The trocars shall receive biological evaluation in accordance with the stipulations of
the series standards of GB/T 16886, and there shall be no biocompatibility hazards.
inserted into the fit clearance. It shall comply with the stipulations of 4.6.2.
5.6.3 Imitate the action of use. Conduct visual observation. It shall comply with the
stipulations of 4.6.3.
5.7 Connection Firmness Test
5.7.1 At the junction of the detachable sheath cap and the puncturing sleeve, apply an
axial static tensile force of 50 N to any component consecutively for 10 s. It shall comply
with the stipulations of 4.7.1.
5.7.2 Imitate the action of use; fix the casing; rotate and draw the casing seat. It shall
comply with the stipulations of 4.7.2.
5.7.3 At the junction of the puncturing lever, apply an axial static tensile force of 15 N
to any component consecutively for 10 s. It shall comply with the stipulations of 4.7.3.
5.8 Air Chocking and Sealing Performance Test
The test method is shown in Appendix A. It shall comply with the stipulations of 4.8.
5.9 Connector of Air Injection Valve
The Luer locking connecting of the air injection valve shall be tested in accordance
with GB/T 1962.2. It shall comply with the stipulations of 4.9.
5.10 Puncturing and Plugging / Unplugging Performance
The evaluation and test methods for the puncturing and plugging / unplugging
performance are shown in Appendix B and Appendix C.
NOTE: the method in the appendix is only a test method for the uniform evaluation of the
puncturing performance and plugging / unplugging performance of the trocars.
5.11 Corrosion Resistance
Corrosion resistanc...
Get QUOTATION in 1-minute: Click YY/T 1710-2020
Historical versions: YY/T 1710-2020
Preview True-PDF (Reload/Scroll if blank)
YY/T 1710-2020: Disposable abdominal trocars
YY/T 1710-2020
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Disposable Abdominal Trocars
ISSUED ON: FEBRUARY 26, 2020
IMPLEMENTED ON: MARCH 1, 2021
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Structure and Materials ... 5
4 Requirements... 7
5 Test Methods ... 10
6 Type Inspection ... 13
7 Labeling and Instruction Manual ... 13
8 Packaging ... 14
Appendix A (normative) Test Method for Air Chocking and Sealing Performance
... 15
Appendix B (informative) Evaluation and Test Method for Puncturing
Performance ... 17
Appendix C (informative) Evaluation and Test Method for Plugging / Unplugging
Performance ... 19
Disposable Abdominal Trocars
1 Scope
This Standard specifies the structure, materials, requirements, test methods, type
inspection, labeling, instruction manual and packaging of disposable abdominal trocars.
This Standard is applicable to disposable abdominal trocars (hereinafter referred to as
trocars) that puncture human abdominal wall tissues during laparoscopic surgery to
establish artificial pneumoperitoneum and operate surgical instrument channel.
2 Normative References
The following documents are indispensable to the application of this document. In
terms of references with a specified date, only versions with a specified date are
applicable to this document. In terms of references without a specified date, the latest
version (including all the modifications) is applicable to this document.
GB/T 1220 Stainless Steel Bars
GB/T 1962.2 Conical Fittings with a 6% (Luer) Taper for Syringes, Needles and Certain
Other Medical Equipment - Part 2: Lock Fittings
GB/T 3280 Cold Rolled Stainless Steel Plate, Sheet and Strip
GB/T 4340.1 Metallic Materials - Vickers Hardness Test - Part 1: Test Method
GB/T 6682-2008 Water for Analytical Laboratory Use - Specification and Test Methods
GB/T 12672 Acrylonitrile-butadiene-styrene (ABS) Resin
GB/T 14233.1-2008 Test Methods for Infusion, Transfusion, Injection Equipment for
Medical Use - Part 1: Chemical Analysis Methods
GB/T 16886 (all parts) Biological Evaluation of Medical Devices
YY/T 0149-2006 Medical Instruments of Stainless Steel - Test Methods of Corrosion
Resistance
YY/T 0466.1 Medical Devices - Symbols to be Used with Medical Device Labels,
Labelling and Information to be Supplied - Part 1: General Requirements
YY/T 0806 Polycarbonate Material for Manufacture of Infusion, Transfusion and
Injection Equipment for Medical Use and Other Medical Devices
4 Requirements
4.1 Appearance
4.1.1 The outer surface of the trocars shall be smooth and clean. There shall be no
defects, such as: burrs, bubbles, impurities, cracks and sintered substances, etc.
4.1.2 The surface of the trocars shall not have visible accumulation of lubricant.
4.1.3 The specification marking on the body of the trocars shall be clearly visible.
4.1.4 If there is a puncturing knife at the tip of the puncturing lever, then, the puncturing
knife shall be flat, and there shall be no rust, sharp edges, burrs or obvious pitting. The
cutting edge of the puncturing knife shall be free of nicks, white edges, wire edges and
cracks, etc.
4.2 Dimensions
The inner diameter and working length L of the puncturing sleeve casing of the
trocars shall comply with the stipulations of Table 1.
4.3 Surface Roughness
If there is a puncturing knife at the tip of the puncturing lever, then, the surface
roughness of the cutting edge surface of the puncturing knife Ra ≤ 0.8 μm.
4.4 Hardness
If there is a skin-puncturing knife at the tip of the puncturing lever, then, the puncturing
knife shall receive thermal treatment; its hardness shall be not less than 650HV10.
4.5 Flexibility
4.5.1 The air injection valve of the trocars shall be able to be flexibly opened and closed;
there shall be no obstruction or jamming.
4.5.2 If the sheath cap is detachable, then, its assembly and disassembly shall be
flexible and convenient; there shall be no obstruction or jamming.
4.5.3 If there is a puncturing knife at the tip of the puncturing lever, then, the puncturing
knife shall be able to be flexibly retracted; there shall be no obstruction or jamming.
4.6 Coordination Performance
4.6.1 The puncturing sleeve and the puncturing lever shall properly coordinate with
each other. There shall be no jamming when plugging or unplugging.
shall be not greater than 10 μg/g.
4.14 Dissolved Precipitates of Polymer Materials of Parts in Contact with
Patient
4.14.1 Appearance (turbidity and color)
The dissolution liquid shall be colorless and transparent, and there shall be no visible
foreign objects.
4.14.2 pH
The pH difference between the dissolution liquid and the blank control solution of the
same batch shall be ≤ 2.0.
4.14.3 Heavy metals
The total content of heavy metals that can be dissolved in the dissolution liquid shall
be ≤ 5 μg/mL.
4.14.4 Reducing substance
Compare the dissolution liquid with the same batch of blank control solution of an equal
volume; the difference in the amount of consumed potassium permanganate solution
[c (1/5KMnO4) = 0.01 mol/L] shall be ≤ 2.0 mL.
4.14.5 Evaporation residue
The total dry residue of the dissolution liquid shall be ≤ 2.0 mg.
4.15 Package Marking and Instruction Manual
4.15.1 The single package of the trocars shall have a marking that complies with the
stipulations of 3.1.2.
4.15.2 The instruction manual of the trocars shall include the specifications of devices
that can be used together with the trocars.
4.15.3 If the puncturing knife has the function of skin-puncturing, then, it shall be clearly
described in the instruction manual.
4.16 Biological Evaluation
The trocars shall receive biological evaluation in accordance with the stipulations of
the series standards of GB/T 16886, and there shall be no biocompatibility hazards.
inserted into the fit clearance. It shall comply with the stipulations of 4.6.2.
5.6.3 Imitate the action of use. Conduct visual observation. It shall comply with the
stipulations of 4.6.3.
5.7 Connection Firmness Test
5.7.1 At the junction of the detachable sheath cap and the puncturing sleeve, apply an
axial static tensile force of 50 N to any component consecutively for 10 s. It shall comply
with the stipulations of 4.7.1.
5.7.2 Imitate the action of use; fix the casing; rotate and draw the casing seat. It shall
comply with the stipulations of 4.7.2.
5.7.3 At the junction of the puncturing lever, apply an axial static tensile force of 15 N
to any component consecutively for 10 s. It shall comply with the stipulations of 4.7.3.
5.8 Air Chocking and Sealing Performance Test
The test method is shown in Appendix A. It shall comply with the stipulations of 4.8.
5.9 Connector of Air Injection Valve
The Luer locking connecting of the air injection valve shall be tested in accordance
with GB/T 1962.2. It shall comply with the stipulations of 4.9.
5.10 Puncturing and Plugging / Unplugging Performance
The evaluation and test methods for the puncturing and plugging / unplugging
performance are shown in Appendix B and Appendix C.
NOTE: the method in the appendix is only a test method for the uniform evaluation of the
puncturing performance and plugging / unplugging performance of the trocars.
5.11 Corrosion Resistance
Corrosion resistanc...
Share