1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1837-2022 English PDF (YY/T1837-2022)
YY/T 1837-2022 English PDF (YY/T1837-2022)
Regular price
$905.00 USD
Regular price
Sale price
$905.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1837-2022 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1837-2022
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1837-2022: Medical electrical equipment - General requirements for reliability
YY/T 1837-2022
Medical electrical equipment - General requirements for reliability
ICS 11.010
CCSC30
People's Republic of China Pharmaceutical Industry Standard
General requirements for reliability of medical electrical equipment
Published on 2022-05-18
2023-06-01 Implementation
Released by the State Drug Administration
directory
Preface III
1 Scope 1
2 Normative references 1
3 Terms and Definitions 1
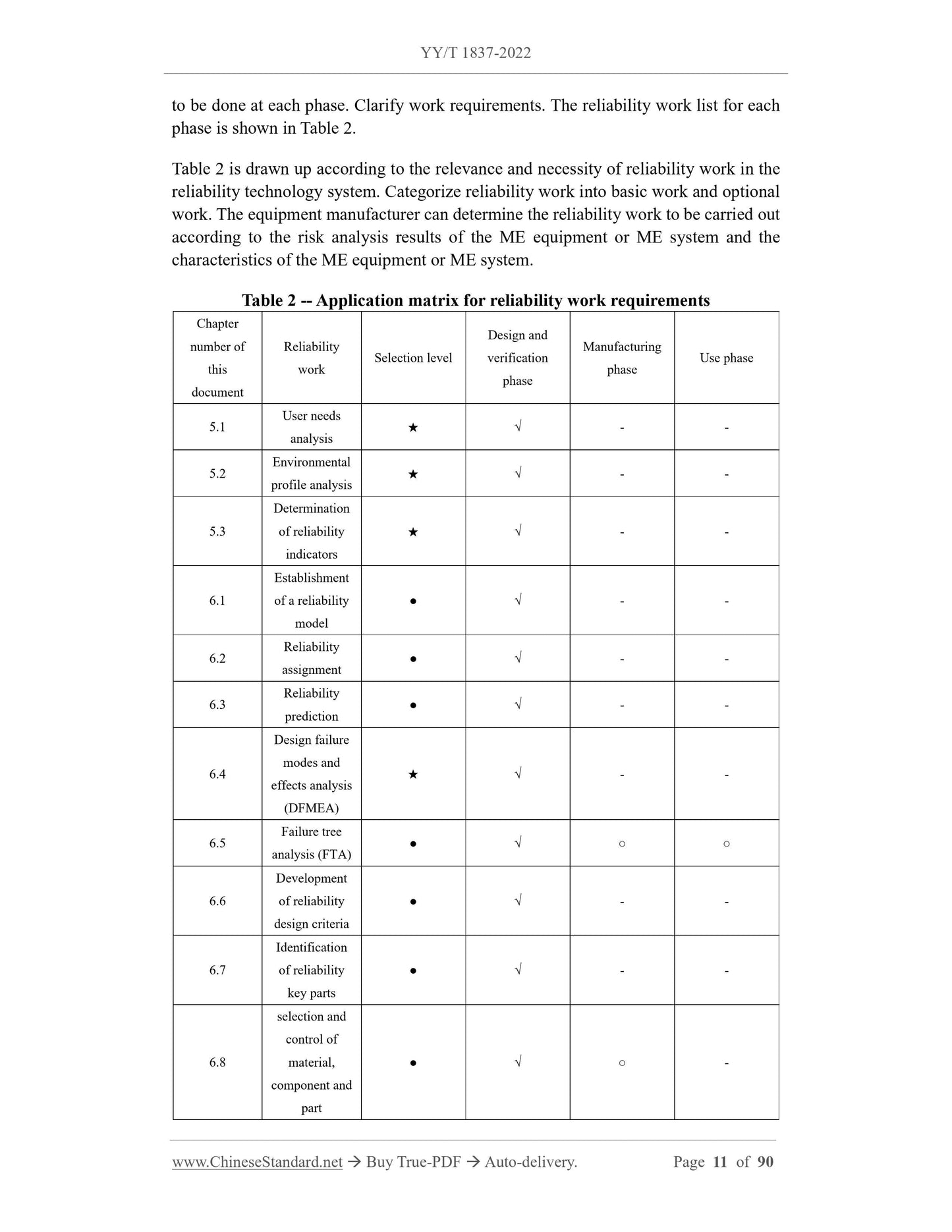
4 General 3
4.1 Reliability work objective 4
4.2 Basic Principles of Reliability Work 4
4.3 Coordination of reliability work with other related work 4
4.4 Reliability work classification 4
4.5 Description of Reliability Work Requirements 4
4.6 Compliance Criteria5
5 Analysis of reliability requirements and determination of requirements 6
5.1 User Requirement Analysis 6
5.2 Environmental profile analysis 6
5.3 Determining reliability indicators7
6 Reliability Design and Analysis 7
6.1 Building a reliability model 7
6.2 Reliability Allocation 7
6.3 Reliability Prediction 7
6.4 Design Failure Modes and Effects Analysis (DFMEA) 8
6.5 Fault Tree Analysis (FTA) 8
6.6 Develop reliability design criteria8
6.7 Identify reliability critical components 9
6.8 Selection and Control of Materials, Components and Components 9
6.9 Finite Element Analysis 9
6.10 Derating Analysis 10
6.11 Circuit Tolerance Analysis 10
6.12 Durability Analysis 10
6.13 Reliability Review 10
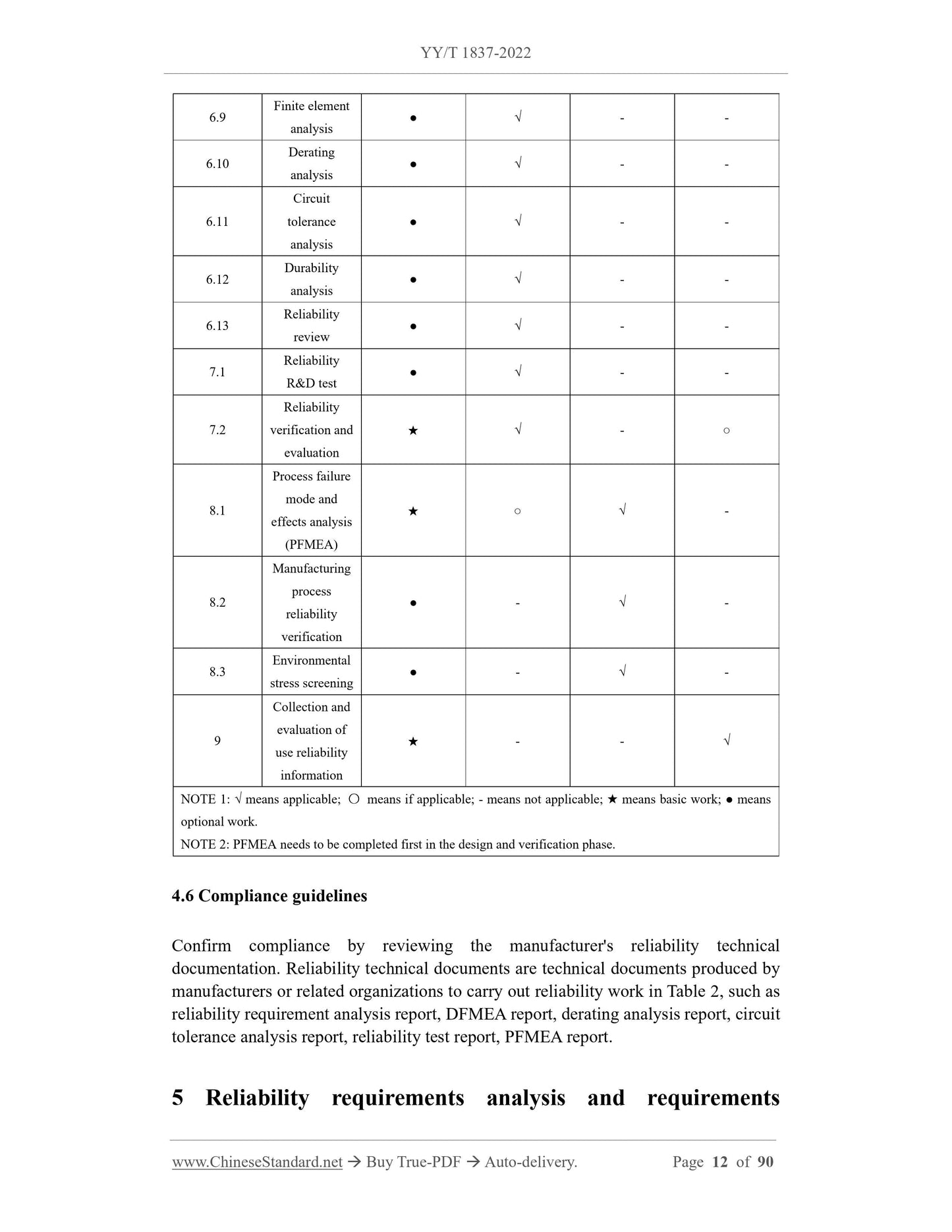
7 Reliability test and evaluation 11
7.1 Reliability R and D Test 11
7.2 Reliability Verification and Evaluation 11
8 Manufacturing Process Reliability 12
8.1 Process Failure Modes and Effects Analysis (PFMEA) 12
8.2 Manufacturing Process Reliability Verification 12
8.3 Environmental stress screening 13
9 Using Reliability Information Collection and Evaluation 13
Appendix A (Informative) Rationale 14
Appendix B (Informative) Reliability Requirements Analysis Form 25
Appendix C (Informative) Environmental Profile Analysis 26
Appendix D (Informative) Reliability Modeling 27
Appendix E (Informative) DFMEA Form Template 31
Appendix F (Informative) Fault Tree FTA Example 32
Appendix G (Informative) Components and Components Selection Reliability Evaluation Table 34
Appendix H (Informative) Finite Element Analysis 35
Appendix I (Informative) Derating Analysis Method 36
Appendix J (Informative) Methods and Procedures for Circuit Tolerance Analysis 39
Appendix K (Informative) Design Review Form Reference Template 43
Appendix L (Informative) Growth Experiments Known to Growth Models 44
Appendix M (Informative) Verification of Reliability Index --- Examples of Routine Test and Accelerated Test Method 49
Appendix N (Informative) Product Failure Cases Introduced by Manufacturing Process 51
Appendix O (Informative) Environmental Stress Screening 52
Reference 54
foreword
This document is in accordance with the provisions of GB/T 1.1-2020 "Guidelines for Standardization Work Part 1.Structure and Drafting Rules of Standardization Documents"
drafted.
Please note that some content of this document may be patented. The issuing agency of this document assumes no responsibility for identifying patents.
This document is proposed by the State Drug Administration.
This document is under the jurisdiction of the National Standardization Technical Committee of Medical Electrical Appliances (SAC/TC10).
This document is drafted by. Shanghai Medical Device Testing Institute, Shenzhen Mindray Biomedical Electronics Co., Ltd., State Drug Administration
Medical Device Technology Evaluation Center of the State Council, Shanghai United Imaging Medical Technology Co., Ltd., Shanghai Siemens Medical Devices Co., Ltd., General Electric
Qi Medical System (China) Co., Ltd., Fresenius Medical Research (Shanghai) Co., Ltd., Shanghai MicroPort Medical Devices (Group) Co., Ltd.,
Chinese People's Liberation Army General Hospital, Philips (China) Investment Co., Ltd.
The main drafters of this document. Yu Hongyi, Shao Lingyun, Yang Pengfei, Zhang Weiqiang, Jia Dongfang, Li Yong, Zhu Jiapeng, Liu Zhenyu, Yu Xiongwei,
He Kunlun, Chen Dayu.
General requirements for reliability of medical electrical equipment
1 Scope
This document specifies the life cycle of medical electrical equipment (hereinafter referred to as ME equipment) and medical electrical systems (hereinafter referred to as ME system).
General requirements and basic methods for carrying out reliability work.
This document applies to the reliability work of various types of ME EQUIPMENT or ME SYSTEMS. This document does not contain relevant information specific to software reliability.
requirements and methods.
Note. All chapters and articles in the main body of this document have corresponding explanations of relevant principles in Appendix A.
2 Normative references
The contents of the following documents constitute essential provisions of this document through normative references in the text. Among them, dated citations
documents, only the version corresponding to that date applies to this document; for undated references, the latest edition (including all amendments) applies to
this document.
GB/T 2900.1-2008 Basic terminology of electrical terminology
GB 9706.1-2020 Medical Electrical Equipment Part 1.General Requirements for Basic Safety and Basic Performance
YY/T 0316-2016 Application of medical device risk management to medical devices
YY/T 1813 Method for collecting and evaluating information on reliability of use of medical electrical equipment
3 Terms and Definitions
GB/T 2900.1-2008, GB 9706.1-2020, YY/T 0316-2016 and the following terms and definitions apply to this
document.
3.1
safety safety
No unacceptable risk to people, property or the environment.
[Source. ISO /IEC GUIDE51.2014, 3.14, with modifications]
3.2
part
The main constituent unit module of the equipment is composed of more than one component.
[Source. GB/T 2900.1-2008, 3.3.18, with modifications]
3.3
Product item
object of consideration.
Note. Products can be individual components, components, equipment, systems.
[Source. GB/T 2900.99-2016, 192-01-01, with modifications]
3.4
risk
A combination of the probability of an injury occurring and the severity of that injury.
Get Quotation: Click YY/T 1837-2022 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1837-2022
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1837-2022: Medical electrical equipment - General requirements for reliability
YY/T 1837-2022
Medical electrical equipment - General requirements for reliability
ICS 11.010
CCSC30
People's Republic of China Pharmaceutical Industry Standard
General requirements for reliability of medical electrical equipment
Published on 2022-05-18
2023-06-01 Implementation
Released by the State Drug Administration
directory
Preface III
1 Scope 1
2 Normative references 1
3 Terms and Definitions 1
4 General 3
4.1 Reliability work objective 4
4.2 Basic Principles of Reliability Work 4
4.3 Coordination of reliability work with other related work 4
4.4 Reliability work classification 4
4.5 Description of Reliability Work Requirements 4
4.6 Compliance Criteria5
5 Analysis of reliability requirements and determination of requirements 6
5.1 User Requirement Analysis 6
5.2 Environmental profile analysis 6
5.3 Determining reliability indicators7
6 Reliability Design and Analysis 7
6.1 Building a reliability model 7
6.2 Reliability Allocation 7
6.3 Reliability Prediction 7
6.4 Design Failure Modes and Effects Analysis (DFMEA) 8
6.5 Fault Tree Analysis (FTA) 8
6.6 Develop reliability design criteria8
6.7 Identify reliability critical components 9
6.8 Selection and Control of Materials, Components and Components 9
6.9 Finite Element Analysis 9
6.10 Derating Analysis 10
6.11 Circuit Tolerance Analysis 10
6.12 Durability Analysis 10
6.13 Reliability Review 10
7 Reliability test and evaluation 11
7.1 Reliability R and D Test 11
7.2 Reliability Verification and Evaluation 11
8 Manufacturing Process Reliability 12
8.1 Process Failure Modes and Effects Analysis (PFMEA) 12
8.2 Manufacturing Process Reliability Verification 12
8.3 Environmental stress screening 13
9 Using Reliability Information Collection and Evaluation 13
Appendix A (Informative) Rationale 14
Appendix B (Informative) Reliability Requirements Analysis Form 25
Appendix C (Informative) Environmental Profile Analysis 26
Appendix D (Informative) Reliability Modeling 27
Appendix E (Informative) DFMEA Form Template 31
Appendix F (Informative) Fault Tree FTA Example 32
Appendix G (Informative) Components and Components Selection Reliability Evaluation Table 34
Appendix H (Informative) Finite Element Analysis 35
Appendix I (Informative) Derating Analysis Method 36
Appendix J (Informative) Methods and Procedures for Circuit Tolerance Analysis 39
Appendix K (Informative) Design Review Form Reference Template 43
Appendix L (Informative) Growth Experiments Known to Growth Models 44
Appendix M (Informative) Verification of Reliability Index --- Examples of Routine Test and Accelerated Test Method 49
Appendix N (Informative) Product Failure Cases Introduced by Manufacturing Process 51
Appendix O (Informative) Environmental Stress Screening 52
Reference 54
foreword
This document is in accordance with the provisions of GB/T 1.1-2020 "Guidelines for Standardization Work Part 1.Structure and Drafting Rules of Standardization Documents"
drafted.
Please note that some content of this document may be patented. The issuing agency of this document assumes no responsibility for identifying patents.
This document is proposed by the State Drug Administration.
This document is under the jurisdiction of the National Standardization Technical Committee of Medical Electrical Appliances (SAC/TC10).
This document is drafted by. Shanghai Medical Device Testing Institute, Shenzhen Mindray Biomedical Electronics Co., Ltd., State Drug Administration
Medical Device Technology Evaluation Center of the State Council, Shanghai United Imaging Medical Technology Co., Ltd., Shanghai Siemens Medical Devices Co., Ltd., General Electric
Qi Medical System (China) Co., Ltd., Fresenius Medical Research (Shanghai) Co., Ltd., Shanghai MicroPort Medical Devices (Group) Co., Ltd.,
Chinese People's Liberation Army General Hospital, Philips (China) Investment Co., Ltd.
The main drafters of this document. Yu Hongyi, Shao Lingyun, Yang Pengfei, Zhang Weiqiang, Jia Dongfang, Li Yong, Zhu Jiapeng, Liu Zhenyu, Yu Xiongwei,
He Kunlun, Chen Dayu.
General requirements for reliability of medical electrical equipment
1 Scope
This document specifies the life cycle of medical electrical equipment (hereinafter referred to as ME equipment) and medical electrical systems (hereinafter referred to as ME system).
General requirements and basic methods for carrying out reliability work.
This document applies to the reliability work of various types of ME EQUIPMENT or ME SYSTEMS. This document does not contain relevant information specific to software reliability.
requirements and methods.
Note. All chapters and articles in the main body of this document have corresponding explanations of relevant principles in Appendix A.
2 Normative references
The contents of the following documents constitute essential provisions of this document through normative references in the text. Among them, dated citations
documents, only the version corresponding to that date applies to this document; for undated references, the latest edition (including all amendments) applies to
this document.
GB/T 2900.1-2008 Basic terminology of electrical terminology
GB 9706.1-2020 Medical Electrical Equipment Part 1.General Requirements for Basic Safety and Basic Performance
YY/T 0316-2016 Application of medical device risk management to medical devices
YY/T 1813 Method for collecting and evaluating information on reliability of use of medical electrical equipment
3 Terms and Definitions
GB/T 2900.1-2008, GB 9706.1-2020, YY/T 0316-2016 and the following terms and definitions apply to this
document.
3.1
safety safety
No unacceptable risk to people, property or the environment.
[Source. ISO /IEC GUIDE51.2014, 3.14, with modifications]
3.2
part
The main constituent unit module of the equipment is composed of more than one component.
[Source. GB/T 2900.1-2008, 3.3.18, with modifications]
3.3
Product item
object of consideration.
Note. Products can be individual components, components, equipment, systems.
[Source. GB/T 2900.99-2016, 192-01-01, with modifications]
3.4
risk
A combination of the probability of an injury occurring and the severity of that injury.
Share