1
/
av
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.1-2021 English PDF (GB1886.1-2021)
GB 1886.1-2021 English PDF (GB1886.1-2021)
Ordinarie pris
$185.00 USD
Ordinarie pris
Försäljningspris
$185.00 USD
Enhetspris
/
per
Frakt beräknas i kassan.
Det gick inte att ladda hämtningstillgänglighet
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.1-2021
Historical versions: GB 1886.1-2021
Preview True-PDF (Reload/Scroll if blank)
GB 1886.1-2021: National food safety standard - Food additives - Sodium carbonate
GB 1886.1-2021
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard -
Food additives - Sodium carbonate
食品添加剂 碳酸钠
ISSUED ON: FEBRUARY 22, 2021
IMPLEMENTED ON: AUGUST 22, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular formula and relative molecular mass ... 4
3 Technical requirements ... 4
Annex A Inspection methods ... 6
National food safety standard -
Food additives - Sodium carbonate
1 Scope
This Standard is applicable to anhydrous sodium carbonate as a food additive
produced by the Hou-Soda method, ammonia-soda method or trona processing
method. At the same time, it is also applicable to the food additive sodium
carbonate decahydrate produced by recrystallization of food additive anhydrous
sodium carbonate.
2 Molecular formula and relative molecular mass
2.1 Molecular formula
Anhydrous sodium carbonate: Na2CO3
Sodium carbonate decahydrate: Na2CO3·10H2O
2.2 Relative molecular mass
Anhydrous sodium carbonate: 105.99 (according to 2018 international relative
atomic mass)
Sodium carbonate decahydrate: 286.14 (according to 2018 international
relative atomic mass)
3 Technical requirements
3.1 Sensory requirements
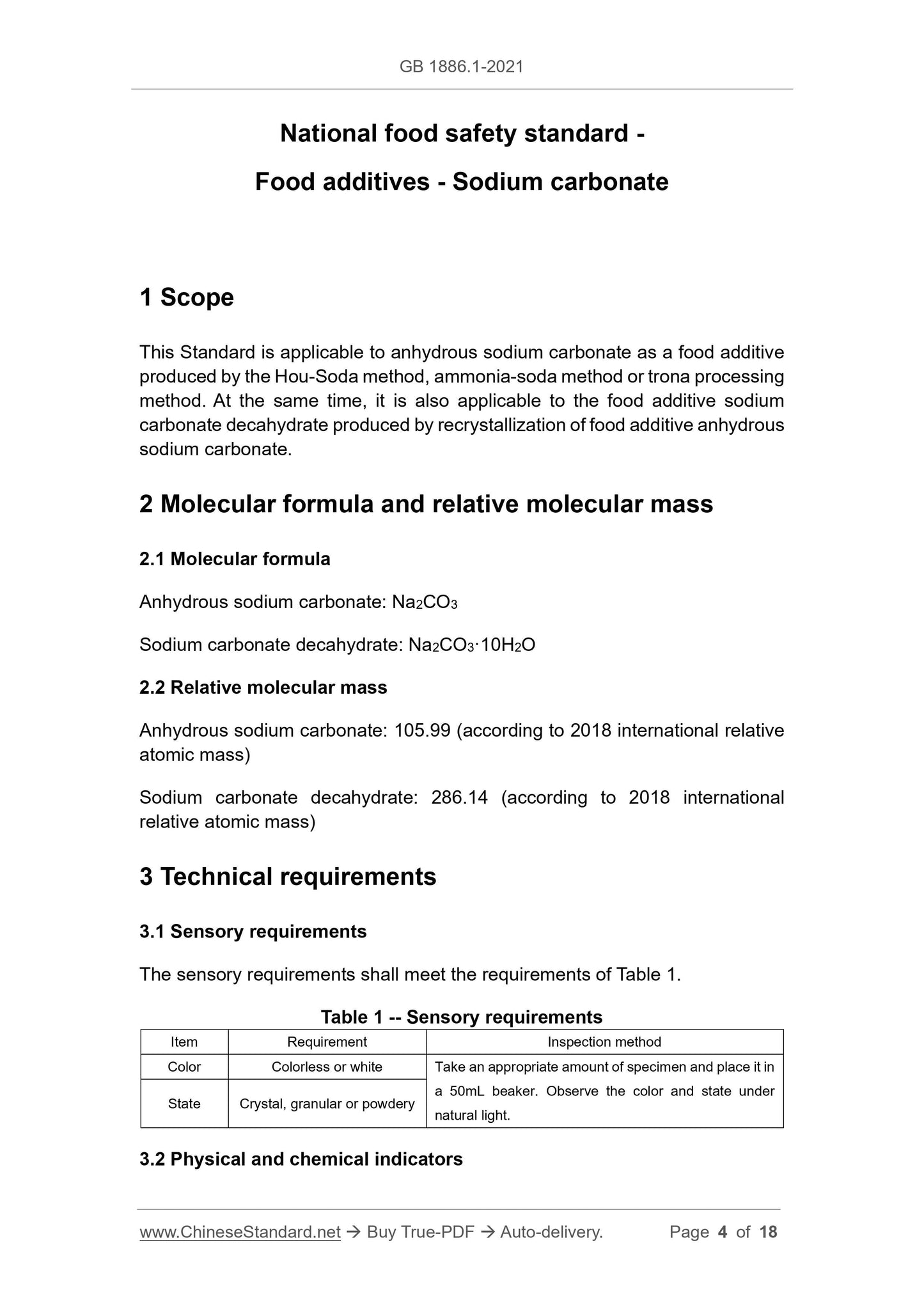
The sensory requirements shall meet the requirements of Table 1.
Table 1 -- Sensory requirements
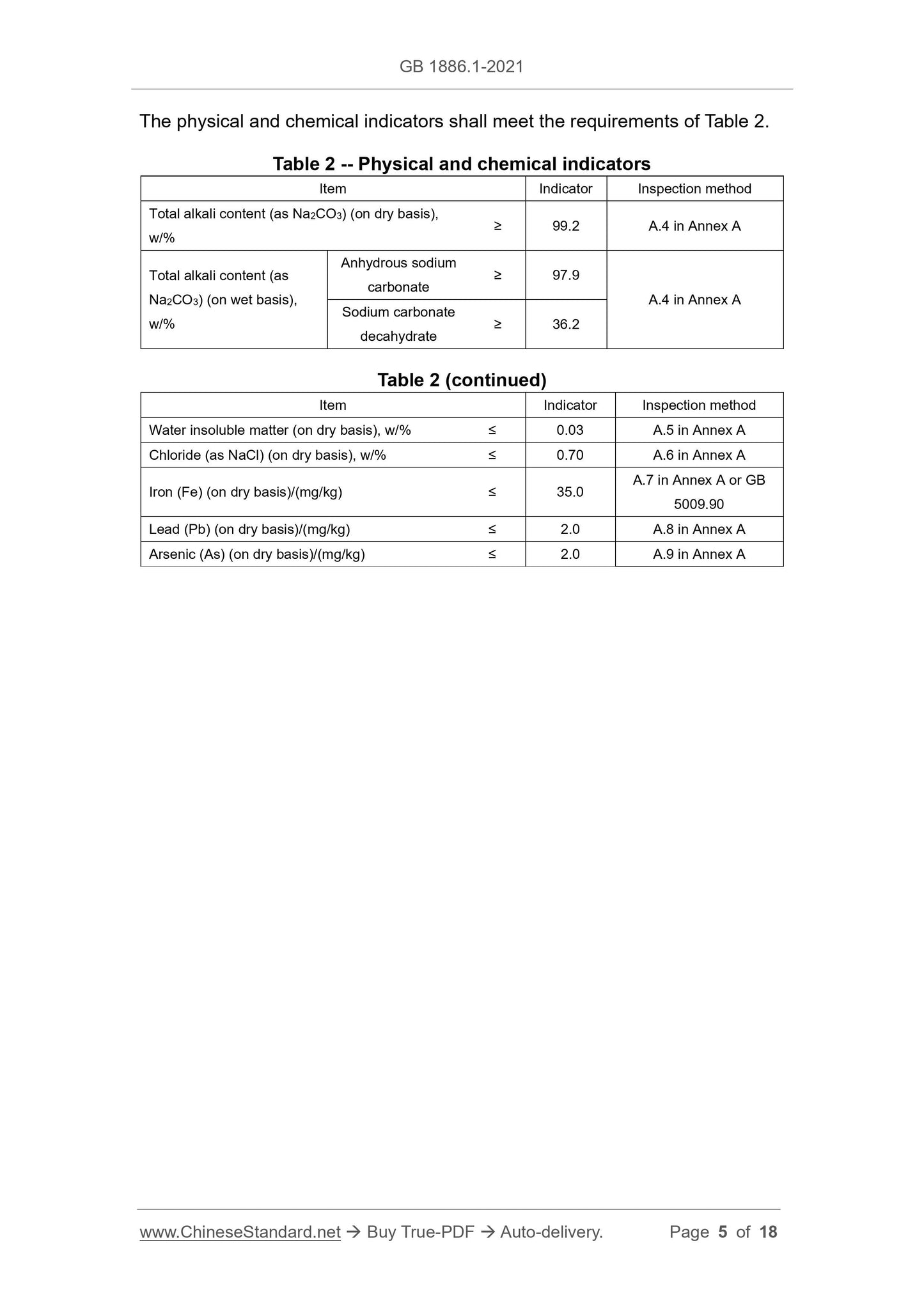
3.2 Physical and chemical indicators
Annex A
Inspection methods
WARNING: Some reagents used in the test method in this Standard are
toxic or corrosive. Be careful when operating! If splashed on the skin,
rinse immediately with water. Severe cases shall be treated immediately.
For reagents containing highly toxic drugs, management shall be strictly
in accordance with relevant regulations. Avoid inhalation or contact with
skin when using. It shall be carried out in a fume hood if necessary. People
with wounds in the exposed area shall not be touched. When using
volatile acid, it shall be carried out in a fume hood. When using flammable
products, it is strictly forbidden to use an open flame for heating.
A.1 General
The reagents and water used in this Standard refer to analytically-pure reagents
and grade three water specified in GB/T 6682 when other requirements are not
indicated. All standard solutions, preparations and products for the
determination of impurities used in the test, when no other requirements are
specified, are prepared according to GB/T 601, GB/T 602, GB/T 603. The
solution used refers to aqueous solution when it is not specified which solvent
is used for preparation.
A.2 Identification test
A.2.1 Reagents and materials
A.2.1.1 Hydrochloric acid.
A.2.1.2 Magnesium sulfate solution: 120g/L.
A.2.1.3 Calcium oxide saturated solution. Preparation: Weigh about 3g of
calcium oxide, to the nearest of 0.1g. Place in a reagent bottle. Add 1000mL of
water. Cap it. After shaking vigorously, place it for clarification. Take the
supernatant when using.
A.2.1.4 Glass rod with platinum wire ring.
A.2.2 Identification methods
A.2.2.1 Preparation of test solution: Weigh about 20g of specimen, to the
nearest of 0.1g. Place in a beaker. Add 100mL of water. Make it dissolved.
A.2.2.2 Use hydrochloric acid to wet the platinum wire ring. Burn to colorless
Where,
m1 - The mass of the specimen and the porcelain crucible before burning, in
grams (g);
m2 - The mass of the specimen and the porcelain crucible after burning, in
grams (g);
m - The specimen mass, in grams.
The test results are based on the arithmetic mean of the parallel determination
results. The absolute difference between two independent determination results
obtained under repeatability conditions is not more than 0.04%.
A.4 Determination of total alkali content (as Na2CO3)
A.4.1 Method summary
Take bromocresol green-methyl red mixed solution as indicator liquid. Use
hydrochloric acid standard titration solution to titrate.
A.4.2 Reagents and materials
A.4.2.1 Hydrochloric acid standard titration solution: c(HCl)=1mol/L.
A.4.2.2 Bromocresol green-methyl red mixed indicator solution.
A.4.3 Analysis steps
A.4.3.1 Determination of total alkali content (on dry basis)
Weigh about 1.7g of specimen that has been burnt to a constant mass
according to A.3, to the nearest of 0.0002g. Place in an Erlenmeyer flask. Use
50mL of water to dissolve the specimen. Add 10 drops of bromocresol green-
methyl red mixed indicator solution. Use hydrochloric acid standard titration
solution to titrate till the solution is from green to dark red. Boil 2min. After
cooling, continue to titrate to dark red as the end point. Conduct blank test at
the same time.
In the blank test, except for not adding the specimen, the other operations and
the type and number of reagents added (except the standard titration solution)
are the same as the determination test.
A.4.3.2 Determination of total alkali content (based on wet basis)
Weigh about 1.7g of anhydrous sodium carbonate or about 4.6g of sodium
carbonate decahydrate specimen, to the nearest of 0.0002g. Place in an
Erlenmeyer flask. Use 50mL of water to dissolve the specimen. Add 10 drops
A.5.2.1 Hydrochloric acid solution: 1+3.
A.5.2.2 Anhydrous sodium carbonate solution: 100g/L.
A.5.2.3 Phenolphthalein indicator solution: 10g/L.
A.5.2.4 Pickling asbestos: Take an appropriate amount of pickled asbestos and
place it in a beaker. Add hydrochloric acid solution. Boil 20min. Use a Buchner
funnel to filter and wash till it is neutral. Take out and soak in sodium carbonate
solution. Boil 20min. Use the Buchner funnel to filter. Use water to wash till it is
neutral (use phenolphthalein solution to inspect). Take it out and place it in a
beaker. Add water to make a paste for future use.
A.5.2.5 Asbestos filter paper.
A.5.3 Instruments and equipment
A.5.3.1 Gooch crucible: Capacity is 30mL.
A.5.3.2 Electric heating constant temperature drying oven: temperature control
range is 110°C±5°C.
A.5.4 Analysis steps
A.5.4.1 Laying of ancient crucible
A.5.4.1.1 Ancient crucible method for pickling asbestos
Place the Gooch crucible on the suction-filtration flask. Spread a layer of pickled
asbestos evenly on the top and bottom of the sieve. During suction-filtration,
use a flat glass rod to press it tightly. Each layer is about 3mm thick. Use
50°C±5°C water to wash till there is no asbestos fiber in the filtrate. Place the
Gooch crucible in an electric heating constant temperature drying box. Dry at
110°C±5°C. Weigh. Repeat washing and drying until the mass is constant.
A.5.4.1.2 Asbestos filter paper ancient crucible method
Place the Gooch crucible on the suction-filtration flask. Lay a layer of asbestos
filter paper under the sieve. Lay two layers of asbestos filter paper on the sieve.
During suction-filtration, use a flat glass rod to press it tightly. Use 50°C±5°C
water to wash to the filter paper. Place the Gooch crucible in an electric heating
constant temperature drying box. Dry at 110°C±5°C. Weigh. Repeat washing
and drying until the mass is constant.
A.5.4.2 Determination
Weigh about 40g of specimen, to the nearest of 0.01g. Put in a beaker. Add
400mL of water at about 40°C to make it dissolved. Keep the solution at
dilute to the scale mark. Shake well.
A.6.1.2.5 Silver nitrate standard titration solution: c(AgNO3)=0.05mol/L.
a) Preparatio...
Get QUOTATION in 1-minute: Click GB 1886.1-2021
Historical versions: GB 1886.1-2021
Preview True-PDF (Reload/Scroll if blank)
GB 1886.1-2021: National food safety standard - Food additives - Sodium carbonate
GB 1886.1-2021
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard -
Food additives - Sodium carbonate
食品添加剂 碳酸钠
ISSUED ON: FEBRUARY 22, 2021
IMPLEMENTED ON: AUGUST 22, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular formula and relative molecular mass ... 4
3 Technical requirements ... 4
Annex A Inspection methods ... 6
National food safety standard -
Food additives - Sodium carbonate
1 Scope
This Standard is applicable to anhydrous sodium carbonate as a food additive
produced by the Hou-Soda method, ammonia-soda method or trona processing
method. At the same time, it is also applicable to the food additive sodium
carbonate decahydrate produced by recrystallization of food additive anhydrous
sodium carbonate.
2 Molecular formula and relative molecular mass
2.1 Molecular formula
Anhydrous sodium carbonate: Na2CO3
Sodium carbonate decahydrate: Na2CO3·10H2O
2.2 Relative molecular mass
Anhydrous sodium carbonate: 105.99 (according to 2018 international relative
atomic mass)
Sodium carbonate decahydrate: 286.14 (according to 2018 international
relative atomic mass)
3 Technical requirements
3.1 Sensory requirements
The sensory requirements shall meet the requirements of Table 1.
Table 1 -- Sensory requirements
3.2 Physical and chemical indicators
Annex A
Inspection methods
WARNING: Some reagents used in the test method in this Standard are
toxic or corrosive. Be careful when operating! If splashed on the skin,
rinse immediately with water. Severe cases shall be treated immediately.
For reagents containing highly toxic drugs, management shall be strictly
in accordance with relevant regulations. Avoid inhalation or contact with
skin when using. It shall be carried out in a fume hood if necessary. People
with wounds in the exposed area shall not be touched. When using
volatile acid, it shall be carried out in a fume hood. When using flammable
products, it is strictly forbidden to use an open flame for heating.
A.1 General
The reagents and water used in this Standard refer to analytically-pure reagents
and grade three water specified in GB/T 6682 when other requirements are not
indicated. All standard solutions, preparations and products for the
determination of impurities used in the test, when no other requirements are
specified, are prepared according to GB/T 601, GB/T 602, GB/T 603. The
solution used refers to aqueous solution when it is not specified which solvent
is used for preparation.
A.2 Identification test
A.2.1 Reagents and materials
A.2.1.1 Hydrochloric acid.
A.2.1.2 Magnesium sulfate solution: 120g/L.
A.2.1.3 Calcium oxide saturated solution. Preparation: Weigh about 3g of
calcium oxide, to the nearest of 0.1g. Place in a reagent bottle. Add 1000mL of
water. Cap it. After shaking vigorously, place it for clarification. Take the
supernatant when using.
A.2.1.4 Glass rod with platinum wire ring.
A.2.2 Identification methods
A.2.2.1 Preparation of test solution: Weigh about 20g of specimen, to the
nearest of 0.1g. Place in a beaker. Add 100mL of water. Make it dissolved.
A.2.2.2 Use hydrochloric acid to wet the platinum wire ring. Burn to colorless
Where,
m1 - The mass of the specimen and the porcelain crucible before burning, in
grams (g);
m2 - The mass of the specimen and the porcelain crucible after burning, in
grams (g);
m - The specimen mass, in grams.
The test results are based on the arithmetic mean of the parallel determination
results. The absolute difference between two independent determination results
obtained under repeatability conditions is not more than 0.04%.
A.4 Determination of total alkali content (as Na2CO3)
A.4.1 Method summary
Take bromocresol green-methyl red mixed solution as indicator liquid. Use
hydrochloric acid standard titration solution to titrate.
A.4.2 Reagents and materials
A.4.2.1 Hydrochloric acid standard titration solution: c(HCl)=1mol/L.
A.4.2.2 Bromocresol green-methyl red mixed indicator solution.
A.4.3 Analysis steps
A.4.3.1 Determination of total alkali content (on dry basis)
Weigh about 1.7g of specimen that has been burnt to a constant mass
according to A.3, to the nearest of 0.0002g. Place in an Erlenmeyer flask. Use
50mL of water to dissolve the specimen. Add 10 drops of bromocresol green-
methyl red mixed indicator solution. Use hydrochloric acid standard titration
solution to titrate till the solution is from green to dark red. Boil 2min. After
cooling, continue to titrate to dark red as the end point. Conduct blank test at
the same time.
In the blank test, except for not adding the specimen, the other operations and
the type and number of reagents added (except the standard titration solution)
are the same as the determination test.
A.4.3.2 Determination of total alkali content (based on wet basis)
Weigh about 1.7g of anhydrous sodium carbonate or about 4.6g of sodium
carbonate decahydrate specimen, to the nearest of 0.0002g. Place in an
Erlenmeyer flask. Use 50mL of water to dissolve the specimen. Add 10 drops
A.5.2.1 Hydrochloric acid solution: 1+3.
A.5.2.2 Anhydrous sodium carbonate solution: 100g/L.
A.5.2.3 Phenolphthalein indicator solution: 10g/L.
A.5.2.4 Pickling asbestos: Take an appropriate amount of pickled asbestos and
place it in a beaker. Add hydrochloric acid solution. Boil 20min. Use a Buchner
funnel to filter and wash till it is neutral. Take out and soak in sodium carbonate
solution. Boil 20min. Use the Buchner funnel to filter. Use water to wash till it is
neutral (use phenolphthalein solution to inspect). Take it out and place it in a
beaker. Add water to make a paste for future use.
A.5.2.5 Asbestos filter paper.
A.5.3 Instruments and equipment
A.5.3.1 Gooch crucible: Capacity is 30mL.
A.5.3.2 Electric heating constant temperature drying oven: temperature control
range is 110°C±5°C.
A.5.4 Analysis steps
A.5.4.1 Laying of ancient crucible
A.5.4.1.1 Ancient crucible method for pickling asbestos
Place the Gooch crucible on the suction-filtration flask. Spread a layer of pickled
asbestos evenly on the top and bottom of the sieve. During suction-filtration,
use a flat glass rod to press it tightly. Each layer is about 3mm thick. Use
50°C±5°C water to wash till there is no asbestos fiber in the filtrate. Place the
Gooch crucible in an electric heating constant temperature drying box. Dry at
110°C±5°C. Weigh. Repeat washing and drying until the mass is constant.
A.5.4.1.2 Asbestos filter paper ancient crucible method
Place the Gooch crucible on the suction-filtration flask. Lay a layer of asbestos
filter paper under the sieve. Lay two layers of asbestos filter paper on the sieve.
During suction-filtration, use a flat glass rod to press it tightly. Use 50°C±5°C
water to wash to the filter paper. Place the Gooch crucible in an electric heating
constant temperature drying box. Dry at 110°C±5°C. Weigh. Repeat washing
and drying until the mass is constant.
A.5.4.2 Determination
Weigh about 40g of specimen, to the nearest of 0.01g. Put in a beaker. Add
400mL of water at about 40°C to make it dissolved. Keep the solution at
dilute to the scale mark. Shake well.
A.6.1.2.5 Silver nitrate standard titration solution: c(AgNO3)=0.05mol/L.
a) Preparatio...
Share