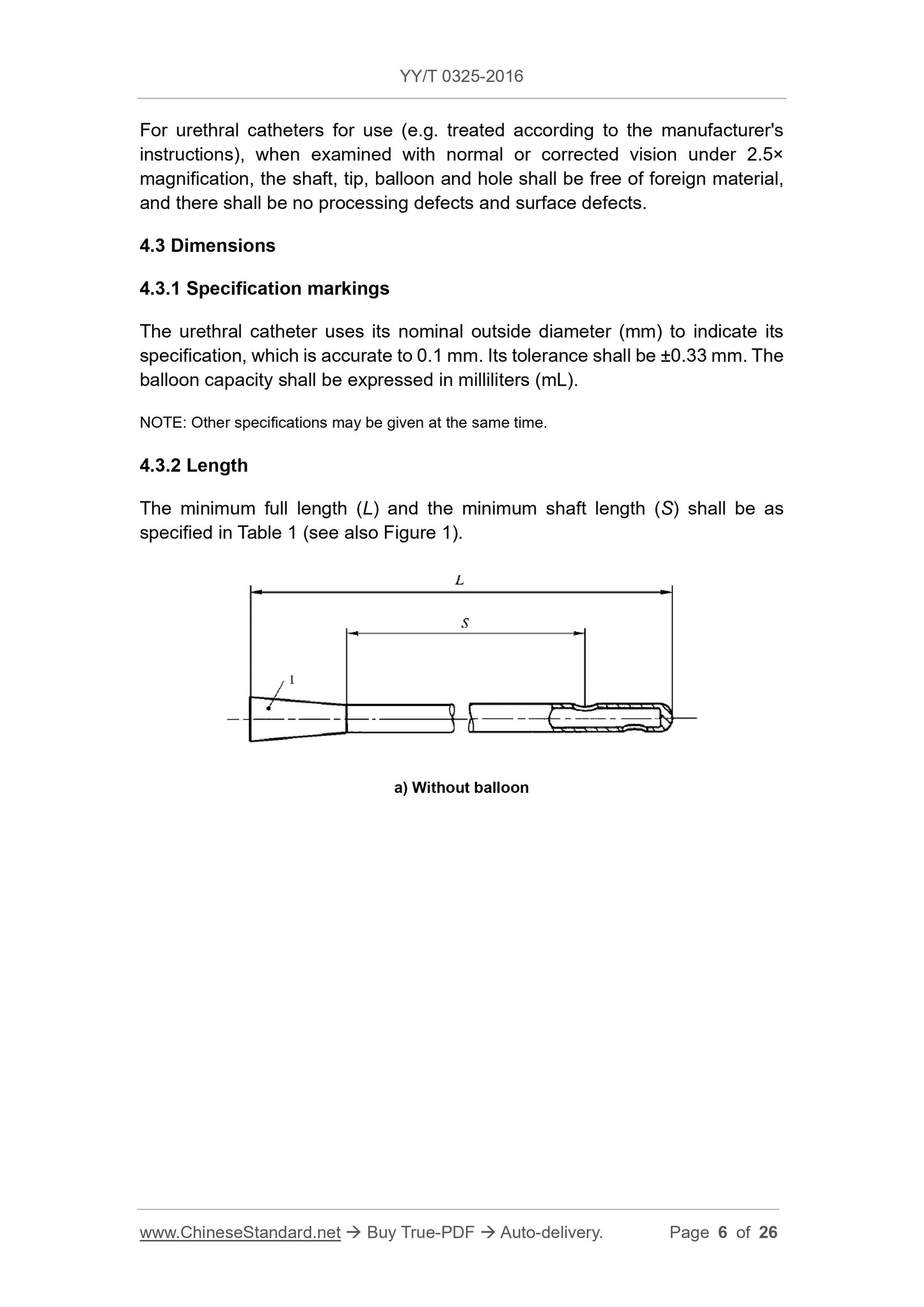
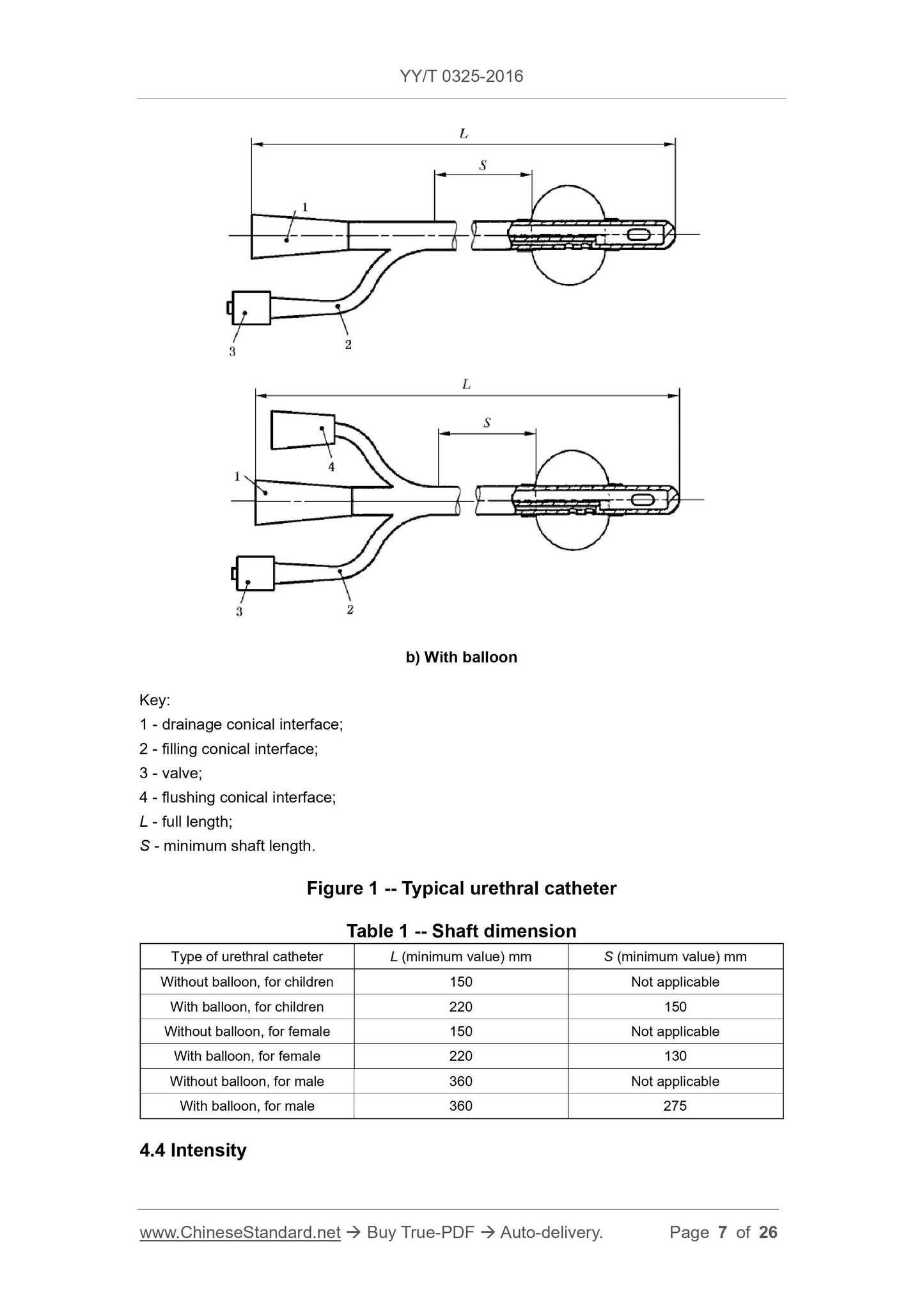
1
/
av
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0325-2016 English PDF (YY0325-2016)
YY 0325-2016 English PDF (YY0325-2016)
Ordinarie pris
$150.00 USD
Ordinarie pris
Försäljningspris
$150.00 USD
Enhetspris
/
per
Frakt beräknas i kassan.
Det gick inte att ladda hämtningstillgänglighet
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY 0325-2016
Historical versions: YY 0325-2016
Preview True-PDF (Reload/Scroll if blank)
YY 0325-2016: Sterile urethral catheter for single use
YY/T 0325-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY/T 0325-2002
Sterile urethral catheter for single use
ISSUED ON. MARCH 23, 2016
IMPLEMENTED ON. JANUARY 01, 2018
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Terms and definitions ... 4
4 Requirements... 5
5 Symbols and markings ... 9
6 Packaging ... 10
Annex A (normative) Test method for determining urethral catheter strength. 11
Annex B (normative) Test method for determining the assembly separation
force of drainage conical interface ... 16
Annex C (normative) Test method for measuring balloon reliability ... 18
Annex D (normative) Test method for determining leakage and/or function of
filling cavity and balloon retraction ... 21
Annex E (normative) Bending resistance test ... 23
Bibliography ... 26
Foreword
All the technical contents of this Standard are mandatory.
This Standard was drafted according to the rules given in GB/T 1.1-2009.
This Standard replaces YY 0325-2002 “Sterile urethral catheter for single use”.
Compared with YY 0325-2005, the main technical differences are as follows.
- MODIFY the normative references (see Clause 2);
- ADD the term. nominal balloon capacity (see 3.5);
- MODIFY “kink resistance” TO “bending resistance performance” (see 4.7
of this Standard, 4.7 of 2002 edition);
- MODIFY the test method for the flow (see 4.8 of this Standard, 4.8 of 2002
edition);
- MODIFY some contents of the symbols and markings (see Clause 5);
- MODIFY the packaging requirements (see Clause 6).
Attention is drawn to the possibility that some of the elements of this document
may be the subject of patent rights. The drafting authority of this document shall
not be held responsible for identifying any or all such patent rights.
This Standard shall be under the jurisdiction of National Technical Committee
on Medical Infusion Devices of Standardization Administration of China
(SAC/TC 106).
Main drafting organization of this Standard. Shandong Quality Inspection
Center for Medical Devices.
Participating drafting organizations of this Standard. Guangzhou Well Lead
Medical Co., Ltd., Shandong Freda Medical Devices Co., Ltd.
Main drafters of this Standard. Wan Min, Song Jinzi, Wang Yanming, Huang
Kaigen.
This Standard was first issued in January 2002.
Sterile urethral catheter for single use
1 Scope
This Standard specifies the terms and definitions, requirements, symbols and
markings, packaging, etc. for sterile urethral catheter for single use.
This Standard applies to sterile urethral catheter with balloon and without
balloon for single use.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 1962.1 Conical fittings with a 6 % (Luer) taper for syringes, needles
and certain other medical equipment - Part 1. General requirement
GB/T 14233.1 Test methods for infusion, transfusion, injection equipment for
medical use - Part 1. Chemical analysis methods
GB/T 15812.1-2005 Catheters other than intravascular catheters - Part 1.
Test methods for common properties
GB/T 19633.1 Packaging for terminally sterilized medical devices - Part 1.
Requirements for materials, sterile barrier systems and packaging systems
YY/T 0313 Medical polymer products - Requirement for package and
information supplied by manufacturer
YY/T 0466.1 Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1. General requirements
YY/T 0615.1 Requirements for medical devices to be designated
3 Terms and definitions
For the purpose of this document, the following terms and definitions apply.
3.1
Annex A
(normative)
Test method for determining urethral catheter strength
A.1 Principle
Urethral catheters with balloon may be used in situ for long periods of time.
Therefore, for urethral catheters with balloon, the urethral catheter is immersed
in simulated urine for 14 days before the test. For urethral catheters without
balloon, this step may be omitted. A tension is applied to the junction between
the tip and the shaft of the urethral catheter. For urethral catheters with side
holes, tension is applied to the holes; for catheters without side holes, tension
is applied between the shaft and the drainage conical interface. After remove
the tension, check the urethral catheter for signs of destruction.
A.2 Reagents
A.2.1 Simulated urine may use either of the following two formulas.
a) Simulated urine consists of the following components (pH is about 6.6).
The reagents are analytical reagents.
Urea 25.0 g
Sodium chloride 9.0 g
Anhydrous disodium phosphate 2.5 g
Ammonium chloride 3.0 g
Anhydrous potassium dihydrogen phosphate 2.5 g
Creatinine 2.0 g
Sodium sulfite, anhydrous 3.0 g
Distilled water dilute to 1.0 L
Warning. This solution helps the growth of microorganisms. At the end of
the tests described in A.3 and C.3, there is likely to be a large amount of
microorganisms. These tests shall be carried out by trained personnel.
Appropriate preventive measures shall be taken when handling the
soaked urethral catheter and discarding the contaminated solution.
b) Simulated urine consists of the following components (pH is about 5.5 ~
Annex B
(normative)
Test method for determining the assembly
separation force of drainage conical interface
B.1 Principle
CONNECT the specified test connector to the drainage conical interface of the
urethral catheter; APPLY an axial tension; CHECK if the connection is
separated.
B.2 Instruments
B.2.1 Test connector, made of rigid material, dimensions are shown in Figure
B.1a).
B.2.2 Fixture or similar devices, for suspending urethral catheters.
B.2.3 Connection device for weights and test connector. For urethral
catheters with a specification of 3.3 mm or less, the total mass of the connection
device and the weight is 0.75 kg; for urethral catheters with a specification
greater than 3.3 mm, the total mass of the connection device and the weight is
1 kg.
B.2.4 Timer.
B.3 Procedure
The test is performed at (23 ± 2) °C.
Allow the urethral catheter's drainage conical interface and connector (B.2.1)
to clean and dry.
PLUG the connector into the drainage conical interface to a depth of 10 mm or
more (i.e., reach or exceed the marking on the connector).
SELECT a point from the connection between the drainage conical interface
and the shaft, CLAMP with a clamp (B.2.2), SUSPEND the urethral catheter
(see Figure B.1b)].
HOLD the weight (B.2.3), on the drain cone of the urethral catheter and gently
place the weight down until it is suspended from the urethral catheter. Hold it
for 1 min and observe.
RECORD whether the test connector is separated from the conical interface.
in the range, it shall be filled up to the maximum capacity.
The urethral catheter is immersed in freshly prepared simulated urine (C.2.2)
and placed in the water bath (C.3.3) controlled at (37 ± 2) °C, to completely
immerse the tip and the balloon.
REMOVE the urethral catheter after being immersed for 14 days in simulated
...
Get QUOTATION in 1-minute: Click YY 0325-2016
Historical versions: YY 0325-2016
Preview True-PDF (Reload/Scroll if blank)
YY 0325-2016: Sterile urethral catheter for single use
YY/T 0325-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY/T 0325-2002
Sterile urethral catheter for single use
ISSUED ON. MARCH 23, 2016
IMPLEMENTED ON. JANUARY 01, 2018
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Terms and definitions ... 4
4 Requirements... 5
5 Symbols and markings ... 9
6 Packaging ... 10
Annex A (normative) Test method for determining urethral catheter strength. 11
Annex B (normative) Test method for determining the assembly separation
force of drainage conical interface ... 16
Annex C (normative) Test method for measuring balloon reliability ... 18
Annex D (normative) Test method for determining leakage and/or function of
filling cavity and balloon retraction ... 21
Annex E (normative) Bending resistance test ... 23
Bibliography ... 26
Foreword
All the technical contents of this Standard are mandatory.
This Standard was drafted according to the rules given in GB/T 1.1-2009.
This Standard replaces YY 0325-2002 “Sterile urethral catheter for single use”.
Compared with YY 0325-2005, the main technical differences are as follows.
- MODIFY the normative references (see Clause 2);
- ADD the term. nominal balloon capacity (see 3.5);
- MODIFY “kink resistance” TO “bending resistance performance” (see 4.7
of this Standard, 4.7 of 2002 edition);
- MODIFY the test method for the flow (see 4.8 of this Standard, 4.8 of 2002
edition);
- MODIFY some contents of the symbols and markings (see Clause 5);
- MODIFY the packaging requirements (see Clause 6).
Attention is drawn to the possibility that some of the elements of this document
may be the subject of patent rights. The drafting authority of this document shall
not be held responsible for identifying any or all such patent rights.
This Standard shall be under the jurisdiction of National Technical Committee
on Medical Infusion Devices of Standardization Administration of China
(SAC/TC 106).
Main drafting organization of this Standard. Shandong Quality Inspection
Center for Medical Devices.
Participating drafting organizations of this Standard. Guangzhou Well Lead
Medical Co., Ltd., Shandong Freda Medical Devices Co., Ltd.
Main drafters of this Standard. Wan Min, Song Jinzi, Wang Yanming, Huang
Kaigen.
This Standard was first issued in January 2002.
Sterile urethral catheter for single use
1 Scope
This Standard specifies the terms and definitions, requirements, symbols and
markings, packaging, etc. for sterile urethral catheter for single use.
This Standard applies to sterile urethral catheter with balloon and without
balloon for single use.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 1962.1 Conical fittings with a 6 % (Luer) taper for syringes, needles
and certain other medical equipment - Part 1. General requirement
GB/T 14233.1 Test methods for infusion, transfusion, injection equipment for
medical use - Part 1. Chemical analysis methods
GB/T 15812.1-2005 Catheters other than intravascular catheters - Part 1.
Test methods for common properties
GB/T 19633.1 Packaging for terminally sterilized medical devices - Part 1.
Requirements for materials, sterile barrier systems and packaging systems
YY/T 0313 Medical polymer products - Requirement for package and
information supplied by manufacturer
YY/T 0466.1 Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1. General requirements
YY/T 0615.1 Requirements for medical devices to be designated
3 Terms and definitions
For the purpose of this document, the following terms and definitions apply.
3.1
Annex A
(normative)
Test method for determining urethral catheter strength
A.1 Principle
Urethral catheters with balloon may be used in situ for long periods of time.
Therefore, for urethral catheters with balloon, the urethral catheter is immersed
in simulated urine for 14 days before the test. For urethral catheters without
balloon, this step may be omitted. A tension is applied to the junction between
the tip and the shaft of the urethral catheter. For urethral catheters with side
holes, tension is applied to the holes; for catheters without side holes, tension
is applied between the shaft and the drainage conical interface. After remove
the tension, check the urethral catheter for signs of destruction.
A.2 Reagents
A.2.1 Simulated urine may use either of the following two formulas.
a) Simulated urine consists of the following components (pH is about 6.6).
The reagents are analytical reagents.
Urea 25.0 g
Sodium chloride 9.0 g
Anhydrous disodium phosphate 2.5 g
Ammonium chloride 3.0 g
Anhydrous potassium dihydrogen phosphate 2.5 g
Creatinine 2.0 g
Sodium sulfite, anhydrous 3.0 g
Distilled water dilute to 1.0 L
Warning. This solution helps the growth of microorganisms. At the end of
the tests described in A.3 and C.3, there is likely to be a large amount of
microorganisms. These tests shall be carried out by trained personnel.
Appropriate preventive measures shall be taken when handling the
soaked urethral catheter and discarding the contaminated solution.
b) Simulated urine consists of the following components (pH is about 5.5 ~
Annex B
(normative)
Test method for determining the assembly
separation force of drainage conical interface
B.1 Principle
CONNECT the specified test connector to the drainage conical interface of the
urethral catheter; APPLY an axial tension; CHECK if the connection is
separated.
B.2 Instruments
B.2.1 Test connector, made of rigid material, dimensions are shown in Figure
B.1a).
B.2.2 Fixture or similar devices, for suspending urethral catheters.
B.2.3 Connection device for weights and test connector. For urethral
catheters with a specification of 3.3 mm or less, the total mass of the connection
device and the weight is 0.75 kg; for urethral catheters with a specification
greater than 3.3 mm, the total mass of the connection device and the weight is
1 kg.
B.2.4 Timer.
B.3 Procedure
The test is performed at (23 ± 2) °C.
Allow the urethral catheter's drainage conical interface and connector (B.2.1)
to clean and dry.
PLUG the connector into the drainage conical interface to a depth of 10 mm or
more (i.e., reach or exceed the marking on the connector).
SELECT a point from the connection between the drainage conical interface
and the shaft, CLAMP with a clamp (B.2.2), SUSPEND the urethral catheter
(see Figure B.1b)].
HOLD the weight (B.2.3), on the drain cone of the urethral catheter and gently
place the weight down until it is suspended from the urethral catheter. Hold it
for 1 min and observe.
RECORD whether the test connector is separated from the conical interface.
in the range, it shall be filled up to the maximum capacity.
The urethral catheter is immersed in freshly prepared simulated urine (C.2.2)
and placed in the water bath (C.3.3) controlled at (37 ± 2) °C, to completely
immerse the tip and the balloon.
REMOVE the urethral catheter after being immersed for 14 days in simulated
...
Share