1
/
จาก
9
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.252-2016 English PDF (GB1886.252-2016)
GB 1886.252-2016 English PDF (GB1886.252-2016)
ราคาปกติ
$85.00 USD
ราคาปกติ
ราคาโปรโมชัน
$85.00 USD
ราคาต่อหน่วย
/
ต่อ
ค่าจัดส่งที่คำนวณในขั้นตอนการชำระเงิน
ไม่สามารถโหลดความพร้อมในการรับสินค้าด้วยตนเองได้
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.252-2016
Historical versions: GB 1886.252-2016
Preview True-PDF (Reload/Scroll if blank)
GB 1886.252-2016: National food safety standard -- Food Additives -- Iron oxide red
GB 1886.252-2016
GB
NATIONAL STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
National food safety standard
Food Additives - Iron oxide red
ISSUED ON. AUGUST 31, 2016
IMPLEMENTED ON. JANUARY 01, 2017
Issued by. National Health and Family Planning Commission of the PRC.
Table of contents
1 Scope ... 3
2 Chemical name, molecular formula and relative molecular mass ... 3
3 Technical requirements ... 3
Appendix A Testing method ... 5
National Food Safety Standard
Food Additives - Iron oxide red
1 Scope
This standard applies to the food additives iron oxide red as obtained with the
ferrous iron as raw material through the two-step oxidation method and
precipitation.
2 Chemical name, molecular formula and relative
molecular mass
2.1 Chemical name
Ferric oxide
2.2 Molecular formula
Fe2O3
2.3 Relative molecular mass
159.70 (in accordance with the 2011 International Relative Atomic Mass)
3 Technical requirements
3.1 Sensory requirements
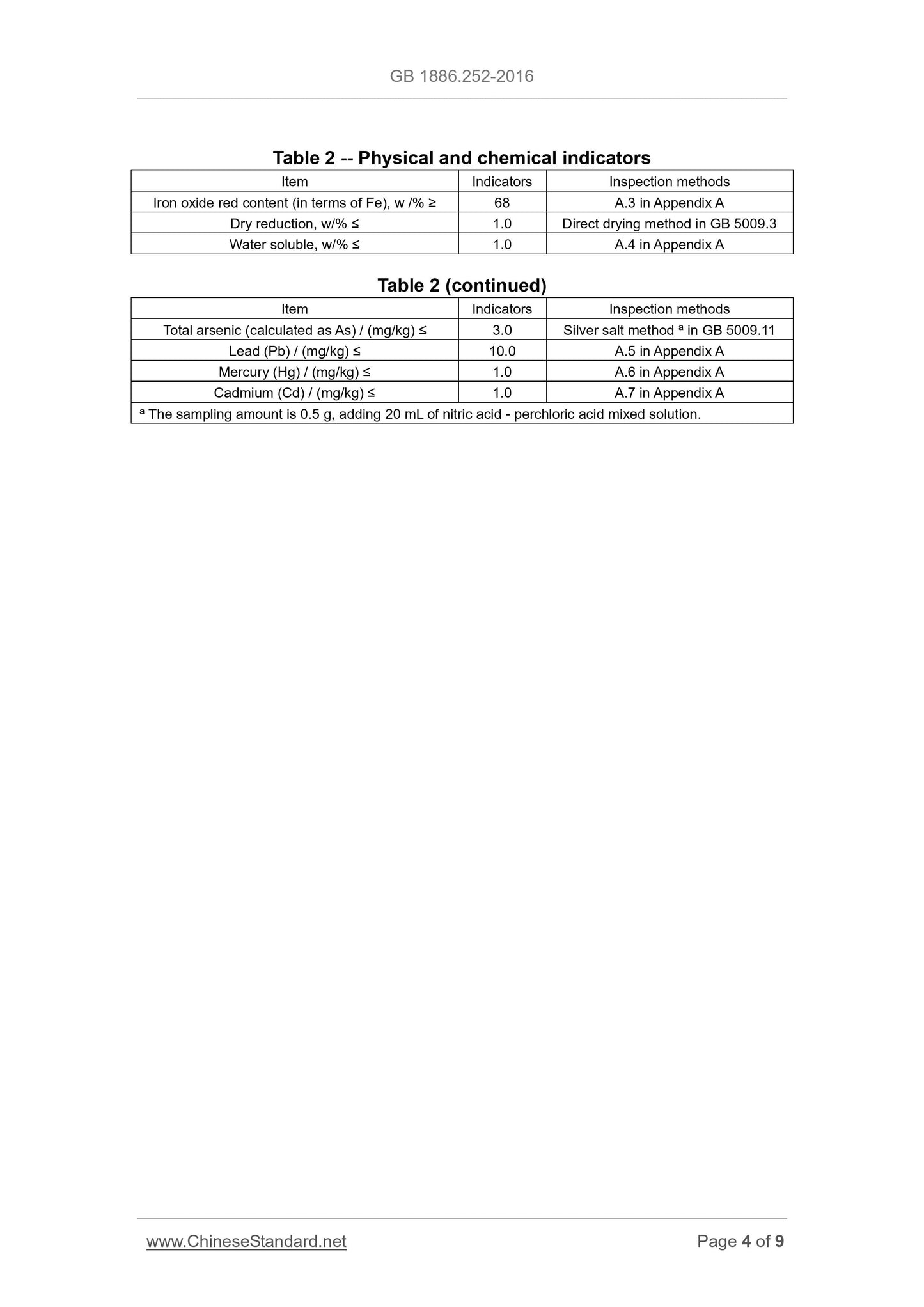
Sensory requirements shall comply with the requirements of Table 1.
Table 1 -- Sensory requirements
Item Requirements Inspection methods
Color Red TAKE appropriate amount of sample and PLACE it uniformly in a white
enamel dish; OBSERVE its color and state under natural light. State Powder
3.2 Physiochemical indicators
Physiochemical indicators shall comply with the provisions of Table 2.
Appendix A
Testing method
A.1 General provisions
Unless otherwise specified in this standard, all the reagents used are of
analytical pure; the standard titration solution, the standard solution for impurity
determination, preparations and products used shall be prepared in
accordance with the provisions of GB/T 601, GB/T 602, and GB/T 603; AND
the test water shall comply with the requirements for level III water as specified
in GB/T 6682. AND the solutions used in the test refer to, unless otherwise
indicated of its solvent, the water solution.
A.2 Identification test
A.2.1 Reagent and solution
A.2.1.1 Hydrochloric acid solution. 1 + 9.
A.2.1.2 Ammonium thiocyanate solution. WEIGH 8 g of ammonium
thiocyanate, accurate to 0.01 g; ADD water to dissolve it and MAKE its volume
reach to 100 mL.
A.2.2 Identification method
WEIGH 0.1 g of sample, accurate to 0.01 g; ADD 5 mL of hydrochloric acid
solution; MAKE it boiling; COOL it down; ADD ammonium thiocyanate solution;
AND it shall in blood red.
A.3 Determination of iron oxide red content (in terms of Fe)
A.3.1 Reagents and materials
A.3.1.1 30% Hydrogen peroxide.
A.3.1.2 Potassium iodide.
A.3.1.3 Hydrochloric acid solution. 9 + 20.
A.3.1.4 Starch indicator solution. TAKE 0.5 g of soluble starch; ADD 5 mL of
water; STIR it uniformly; slowly POUR it into 100 mL of boiling water; STIR it
while adding it; CONTINUE boiling it for 2 min; LET it cool down; TAKE the
A.5.2 Instruments and equipment
Atomic absorption spectrometer.
A.5.3 Analytical procedures
WEIGH about 2 g of the sample, accurate to 0.01 g; PLACE it into a 100 mL
beaker; ADD 15 mL of hydrochloric acid solution; HEAT it on the hot plate to
completely dissolve it; COOL it down, ADD 2 mL of hydrogen peroxide;
CONTINE heating to slightly boil it; MAINTAIN for 3 min; after cooling it down,
ADD 25 mL of 4-methyl-2-pentanone and 10 mL of hydrochloric acid solution;
TRANSFER the solution into a 125 mL separating funnel; SHAKE it for 2 min;
LET it stand for 30 s; TRANSFER the lower layer water phase into the beaker;
HEAT to make the solution concentrate to about 5 mL ~ 10 mL; FILTER the
residual solution; USE water to wash the filtration residue; COMBINE it with
filtrate; MAKE the volume reach to 100 mL; PREPARE for use. USE the same
method to prepare blank solution.
PREPARE the lead standard solution in accordance A.5.1.4, and FOLLOW the
flame atomic absorption spectrometry in accordance with GB 5009.12 to make
determination.
The results of the test are based on the arithmetic mean of the parallel
measurement results. The absolute difference between the two independent
determinations obtained under repeatability shall be not greater than 1.0
mg/kg.
A.6 Determination of mercury (Hg)
A.6.1 Reagents and materials
A.6.1.1 Sodium hydroxide solution. 1 g/L
A.6.1.2 Sodium borohydride solution. 8 g/L (solvent is 1 g/L sodium hydroxide
solution).
A.6.1.3 Mercury standard solution. it is prepared and calibrated in accordance
with GB/T 602, diluted in accordance with the requirements of the instruments
used to prepare it into the standard solutions of mercury concentration in 0.001
μg/mL, 0.002 μg/mL, and 0.003 μg/mL, respectively.
A.6.2 Instruments and equipment
Atomic absorption spectrometer.
A.6.3 Atomic absorption spectrometer reference conditions
Get QUOTATION in 1-minute: Click GB 1886.252-2016
Historical versions: GB 1886.252-2016
Preview True-PDF (Reload/Scroll if blank)
GB 1886.252-2016: National food safety standard -- Food Additives -- Iron oxide red
GB 1886.252-2016
GB
NATIONAL STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
National food safety standard
Food Additives - Iron oxide red
ISSUED ON. AUGUST 31, 2016
IMPLEMENTED ON. JANUARY 01, 2017
Issued by. National Health and Family Planning Commission of the PRC.
Table of contents
1 Scope ... 3
2 Chemical name, molecular formula and relative molecular mass ... 3
3 Technical requirements ... 3
Appendix A Testing method ... 5
National Food Safety Standard
Food Additives - Iron oxide red
1 Scope
This standard applies to the food additives iron oxide red as obtained with the
ferrous iron as raw material through the two-step oxidation method and
precipitation.
2 Chemical name, molecular formula and relative
molecular mass
2.1 Chemical name
Ferric oxide
2.2 Molecular formula
Fe2O3
2.3 Relative molecular mass
159.70 (in accordance with the 2011 International Relative Atomic Mass)
3 Technical requirements
3.1 Sensory requirements
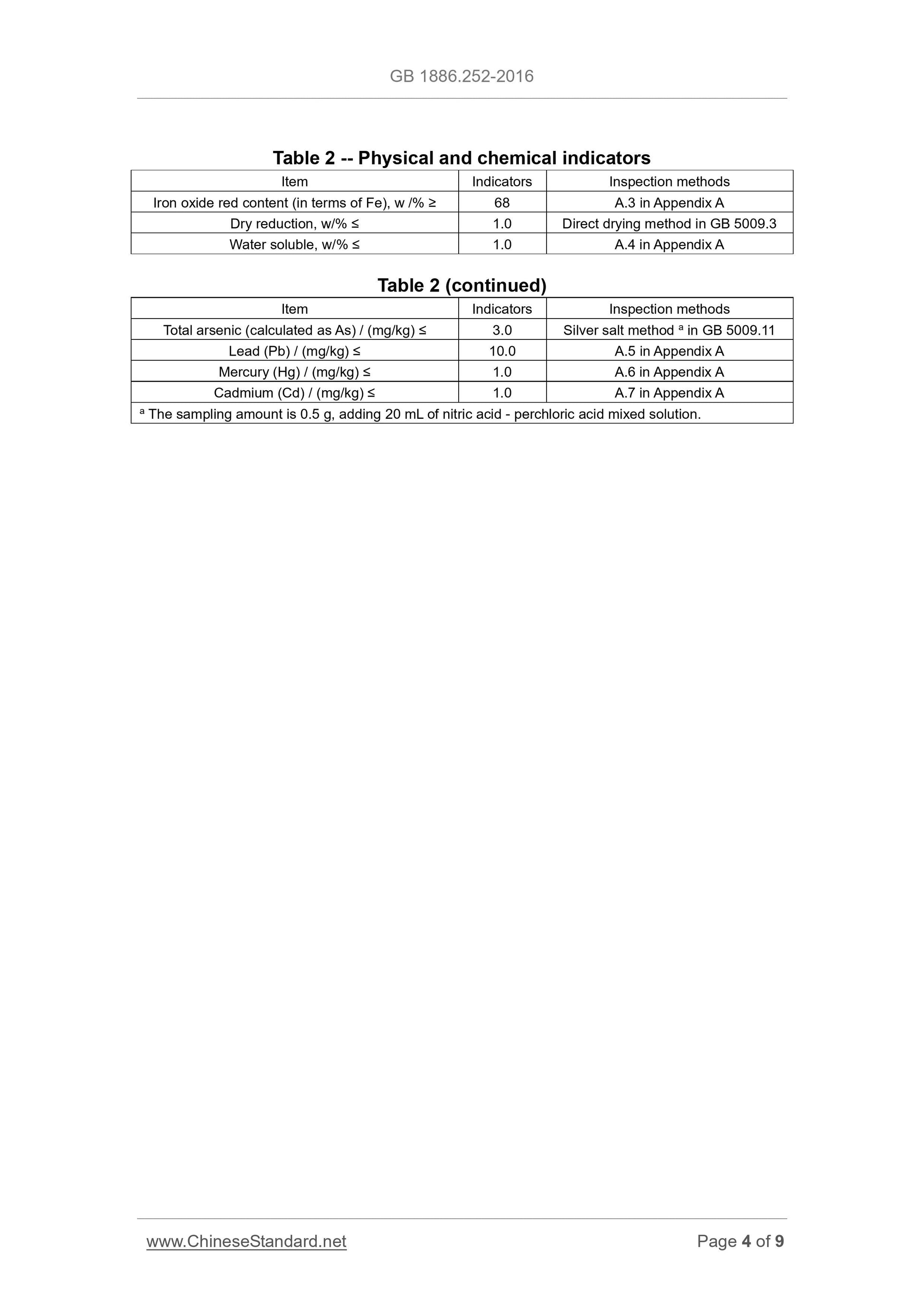
Sensory requirements shall comply with the requirements of Table 1.
Table 1 -- Sensory requirements
Item Requirements Inspection methods
Color Red TAKE appropriate amount of sample and PLACE it uniformly in a white
enamel dish; OBSERVE its color and state under natural light. State Powder
3.2 Physiochemical indicators
Physiochemical indicators shall comply with the provisions of Table 2.
Appendix A
Testing method
A.1 General provisions
Unless otherwise specified in this standard, all the reagents used are of
analytical pure; the standard titration solution, the standard solution for impurity
determination, preparations and products used shall be prepared in
accordance with the provisions of GB/T 601, GB/T 602, and GB/T 603; AND
the test water shall comply with the requirements for level III water as specified
in GB/T 6682. AND the solutions used in the test refer to, unless otherwise
indicated of its solvent, the water solution.
A.2 Identification test
A.2.1 Reagent and solution
A.2.1.1 Hydrochloric acid solution. 1 + 9.
A.2.1.2 Ammonium thiocyanate solution. WEIGH 8 g of ammonium
thiocyanate, accurate to 0.01 g; ADD water to dissolve it and MAKE its volume
reach to 100 mL.
A.2.2 Identification method
WEIGH 0.1 g of sample, accurate to 0.01 g; ADD 5 mL of hydrochloric acid
solution; MAKE it boiling; COOL it down; ADD ammonium thiocyanate solution;
AND it shall in blood red.
A.3 Determination of iron oxide red content (in terms of Fe)
A.3.1 Reagents and materials
A.3.1.1 30% Hydrogen peroxide.
A.3.1.2 Potassium iodide.
A.3.1.3 Hydrochloric acid solution. 9 + 20.
A.3.1.4 Starch indicator solution. TAKE 0.5 g of soluble starch; ADD 5 mL of
water; STIR it uniformly; slowly POUR it into 100 mL of boiling water; STIR it
while adding it; CONTINUE boiling it for 2 min; LET it cool down; TAKE the
A.5.2 Instruments and equipment
Atomic absorption spectrometer.
A.5.3 Analytical procedures
WEIGH about 2 g of the sample, accurate to 0.01 g; PLACE it into a 100 mL
beaker; ADD 15 mL of hydrochloric acid solution; HEAT it on the hot plate to
completely dissolve it; COOL it down, ADD 2 mL of hydrogen peroxide;
CONTINE heating to slightly boil it; MAINTAIN for 3 min; after cooling it down,
ADD 25 mL of 4-methyl-2-pentanone and 10 mL of hydrochloric acid solution;
TRANSFER the solution into a 125 mL separating funnel; SHAKE it for 2 min;
LET it stand for 30 s; TRANSFER the lower layer water phase into the beaker;
HEAT to make the solution concentrate to about 5 mL ~ 10 mL; FILTER the
residual solution; USE water to wash the filtration residue; COMBINE it with
filtrate; MAKE the volume reach to 100 mL; PREPARE for use. USE the same
method to prepare blank solution.
PREPARE the lead standard solution in accordance A.5.1.4, and FOLLOW the
flame atomic absorption spectrometry in accordance with GB 5009.12 to make
determination.
The results of the test are based on the arithmetic mean of the parallel
measurement results. The absolute difference between the two independent
determinations obtained under repeatability shall be not greater than 1.0
mg/kg.
A.6 Determination of mercury (Hg)
A.6.1 Reagents and materials
A.6.1.1 Sodium hydroxide solution. 1 g/L
A.6.1.2 Sodium borohydride solution. 8 g/L (solvent is 1 g/L sodium hydroxide
solution).
A.6.1.3 Mercury standard solution. it is prepared and calibrated in accordance
with GB/T 602, diluted in accordance with the requirements of the instruments
used to prepare it into the standard solutions of mercury concentration in 0.001
μg/mL, 0.002 μg/mL, and 0.003 μg/mL, respectively.
A.6.2 Instruments and equipment
Atomic absorption spectrometer.
A.6.3 Atomic absorption spectrometer reference conditions
Share