1
/
/
10
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.27-2015 English PDF (GB1886.27-2015)
GB 1886.27-2015 English PDF (GB1886.27-2015)
Normal fiyat
$70.00 USD
Normal fiyat
İndirimli fiyat
$70.00 USD
Birim fiyat
/
/
Kargo, ödeme sayfasında hesaplanır.
Teslim alım stok durumu yüklenemedi
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.27-2015
Historical versions: GB 1886.27-2015
Preview True-PDF (Reload/Scroll if blank)
GB 1886.27-2015: National Food Safety Standard -- Food Additives -- Sucrose esters of fatty acid
GB 1886.27-2015
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard -
Food additive - Sucrose esters of fatty acid
食品添加剂 蔗糖脂肪酸酯
ISSUED ON: SEPTEMBER 22, 2015
IMPLEMENTED ON: MARCH 22, 2016
Issued by: National Health and Family Planning Commission of the
People's Republic of China
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular formula ... 4
3 Technical requirements ... 4
Annex A Test methods ... 6
National Food Safety Standard -
Food additive - Sucrose esters of fatty acid
1 Scope
This Standard applies to the food additive - sucrose esters of fatty acid, which
is obtained by esterifying and refining sucrose and edible fats or fatty acids as
main raw materials.
2 Molecular formula
(RCOO)nC12H12O3(OH)8-n
R - hydrocarbon group of fatty acid;
n - hydroxyl esterification number of sucrose.
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall comply with the provisions of Table 1.
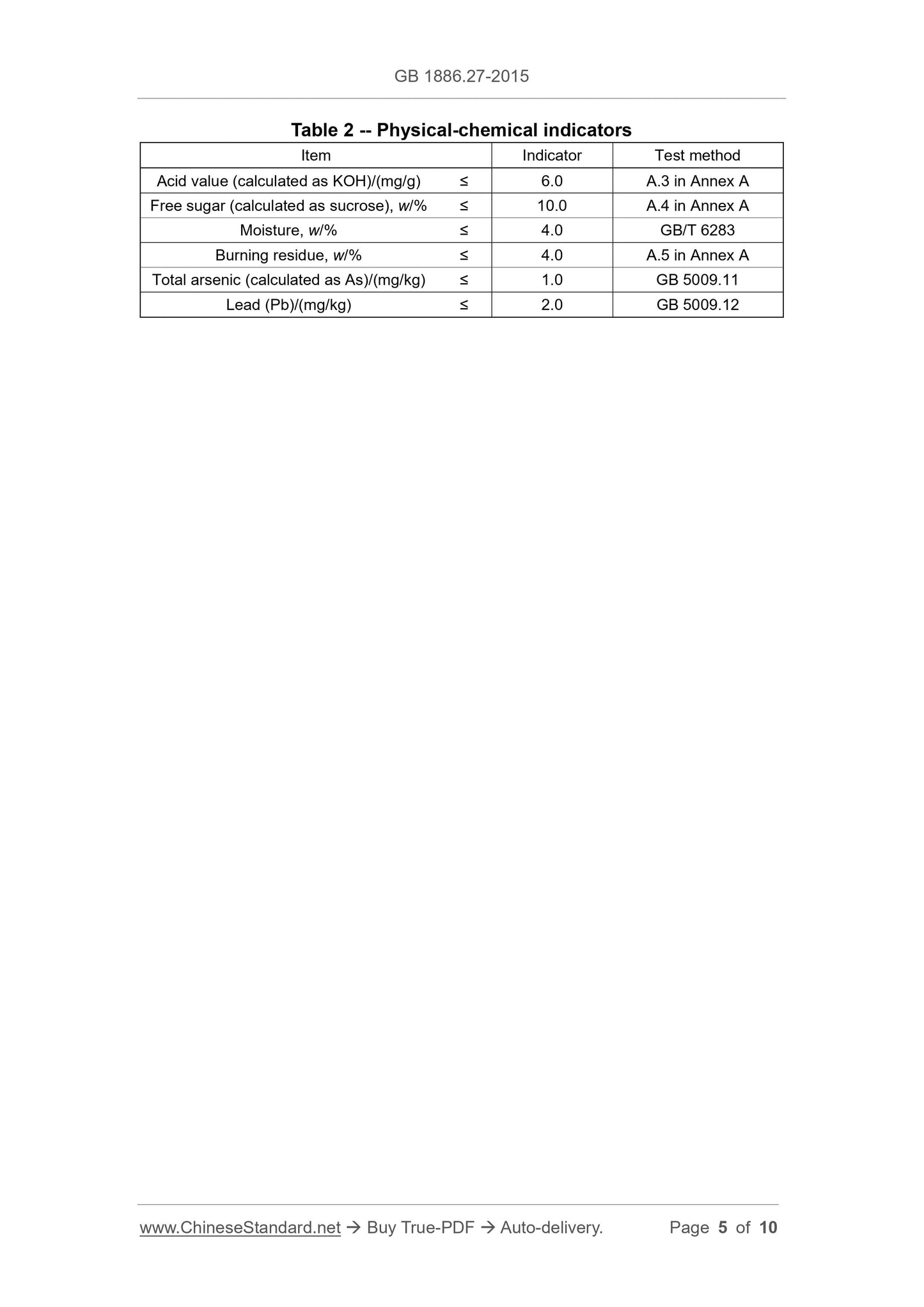
3.2 Physical-chemical indicators
Physical-chemical indicators shall comply with the provisions of Table 2.
Annex A
Test methods
A.1 General
Unless otherwise specified, the reagents and water used in this Standard refer
to analytical reagents and grade 3 water specified in GB/T 6682. Unless
otherwise specified, the standard titration solutions used in the test, the
standard solutions, preparations and products used in the determination of
impurities are prepared according to the provisions of GB/T 601, GB/T 602 and
GB/T 603. When the solution used in the test does not indicate which solvent
is used for preparation, it refers to aqueous solution.
A.2 Identification test
A.2.1 Reagents and materials
A.2.1.1 Sodium chloride.
A.2.1.2 Anhydrous sodium sulfate.
A.2.1.3 Ether.
A.2.1.4 Hydrochloric acid solution: 1 + 3.
A.2.1.5 Potassium hydroxide-ethanol solution.
A.2.1.6 Anthrone sulfuric acid solution: 2 g/L.
A.2.2 Analysis steps
A.2.2.1 Sample processing
WEIGH 1 g of sample in a 250 mL Erlenmeyer flask; ADD 25 mL of potassium
hydroxide-ethanol solution; INSTALL a reflux condenser; HEAT on a water bath
and slightly BOIL for 1 h; REMOVE after a little cooling; ADD 50 mL of water;
HEAT to concentrate to about 30 mL; ADD 10 mL of hydrochloric acid solution;
SHAKE thoroughly; ADD sodium chloride to make it a saturated solution;
SHAKE well; TRANSFER to a separatory funnel; EXTRACT twice with 30 mL
of diethyl ether each time; SEPARATE the ether layer and the water layer, for
test.
A.2.2.2 Identification
After the ether layer is washed with 20 mL of saturated sodium chloride solution,
moles per liter (mol/L);
M - the molar mass of potassium hydroxide, in grams per mole (g/mol),
[M(KOH) = 56.1];
m1 - the mass of the sample, in grams (g).
The test result is based on the arithmetic mean of the two parallel determination
results (retain one decimal place). The absolute difference between two
independent determination results obtained under repeatability conditions shall
not exceed 5 % of the arithmetic mean.
A.4 Determination of free sugar (calculated as sucrose)
A.4.1 Reagents and materials
A.4.1.1 n-butanol.
A.4.1.2 Sodium chloride solution: 50 g/L.
A.4.1.3 Hydrochloric acid solution: 6 mol/L. TAKE 540 mL of hydrochloric acid;
DILUTE to 1000 mL with water; SHAKE well.
A.4.1.4 Sodium hydroxide solution: 200 g/L.
A.4.1.5 Glucose standard solution: WEIGH 1.000 g, to the nearest 0.0001 g, of
pure glucose that has been dried to constant weight at 98 °C ~ 100 °C; after
adding water to dissolve, ADD 5 mL of hydrochloric acid; DILUTE to 200 mL
with water.
A.4.1.6 Fehling's solution A.
A.4.1.7 Fehling's solution B.
A.4.1.8 Methyl red indicator solution: 1 g/L.
A.4.1.9 Methylene blue indicator solution: 0.05 g/L.
A.4.2 Analysis steps
A.4.2.1 Sample processing
WEIGH about 2 g of sample (to the nearest 0.01 g); PLACE it in an Erlenmeyer
flask; ADD 40 mL of n-butanol; HEAT to dissolve in a water bath. TRANSFER
to a 125 mL separatory funnel; EXTRACT twice with 10 mL of sodium chloride
solution at 60 °C to 70 °C each time; SEPARATE (centrifuge if necessary);
COMBINE the extracts. ADD 2.0 mL of 6 mol/L hydrochloric acid solution; HEAT
in a water bath at 68 °C ~ 70 °C for 15 min; ADD methyl red indicator solution
Get QUOTATION in 1-minute: Click GB 1886.27-2015
Historical versions: GB 1886.27-2015
Preview True-PDF (Reload/Scroll if blank)
GB 1886.27-2015: National Food Safety Standard -- Food Additives -- Sucrose esters of fatty acid
GB 1886.27-2015
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard -
Food additive - Sucrose esters of fatty acid
食品添加剂 蔗糖脂肪酸酯
ISSUED ON: SEPTEMBER 22, 2015
IMPLEMENTED ON: MARCH 22, 2016
Issued by: National Health and Family Planning Commission of the
People's Republic of China
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular formula ... 4
3 Technical requirements ... 4
Annex A Test methods ... 6
National Food Safety Standard -
Food additive - Sucrose esters of fatty acid
1 Scope
This Standard applies to the food additive - sucrose esters of fatty acid, which
is obtained by esterifying and refining sucrose and edible fats or fatty acids as
main raw materials.
2 Molecular formula
(RCOO)nC12H12O3(OH)8-n
R - hydrocarbon group of fatty acid;
n - hydroxyl esterification number of sucrose.
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall comply with the provisions of Table 1.
3.2 Physical-chemical indicators
Physical-chemical indicators shall comply with the provisions of Table 2.
Annex A
Test methods
A.1 General
Unless otherwise specified, the reagents and water used in this Standard refer
to analytical reagents and grade 3 water specified in GB/T 6682. Unless
otherwise specified, the standard titration solutions used in the test, the
standard solutions, preparations and products used in the determination of
impurities are prepared according to the provisions of GB/T 601, GB/T 602 and
GB/T 603. When the solution used in the test does not indicate which solvent
is used for preparation, it refers to aqueous solution.
A.2 Identification test
A.2.1 Reagents and materials
A.2.1.1 Sodium chloride.
A.2.1.2 Anhydrous sodium sulfate.
A.2.1.3 Ether.
A.2.1.4 Hydrochloric acid solution: 1 + 3.
A.2.1.5 Potassium hydroxide-ethanol solution.
A.2.1.6 Anthrone sulfuric acid solution: 2 g/L.
A.2.2 Analysis steps
A.2.2.1 Sample processing
WEIGH 1 g of sample in a 250 mL Erlenmeyer flask; ADD 25 mL of potassium
hydroxide-ethanol solution; INSTALL a reflux condenser; HEAT on a water bath
and slightly BOIL for 1 h; REMOVE after a little cooling; ADD 50 mL of water;
HEAT to concentrate to about 30 mL; ADD 10 mL of hydrochloric acid solution;
SHAKE thoroughly; ADD sodium chloride to make it a saturated solution;
SHAKE well; TRANSFER to a separatory funnel; EXTRACT twice with 30 mL
of diethyl ether each time; SEPARATE the ether layer and the water layer, for
test.
A.2.2.2 Identification
After the ether layer is washed with 20 mL of saturated sodium chloride solution,
moles per liter (mol/L);
M - the molar mass of potassium hydroxide, in grams per mole (g/mol),
[M(KOH) = 56.1];
m1 - the mass of the sample, in grams (g).
The test result is based on the arithmetic mean of the two parallel determination
results (retain one decimal place). The absolute difference between two
independent determination results obtained under repeatability conditions shall
not exceed 5 % of the arithmetic mean.
A.4 Determination of free sugar (calculated as sucrose)
A.4.1 Reagents and materials
A.4.1.1 n-butanol.
A.4.1.2 Sodium chloride solution: 50 g/L.
A.4.1.3 Hydrochloric acid solution: 6 mol/L. TAKE 540 mL of hydrochloric acid;
DILUTE to 1000 mL with water; SHAKE well.
A.4.1.4 Sodium hydroxide solution: 200 g/L.
A.4.1.5 Glucose standard solution: WEIGH 1.000 g, to the nearest 0.0001 g, of
pure glucose that has been dried to constant weight at 98 °C ~ 100 °C; after
adding water to dissolve, ADD 5 mL of hydrochloric acid; DILUTE to 200 mL
with water.
A.4.1.6 Fehling's solution A.
A.4.1.7 Fehling's solution B.
A.4.1.8 Methyl red indicator solution: 1 g/L.
A.4.1.9 Methylene blue indicator solution: 0.05 g/L.
A.4.2 Analysis steps
A.4.2.1 Sample processing
WEIGH about 2 g of sample (to the nearest 0.01 g); PLACE it in an Erlenmeyer
flask; ADD 40 mL of n-butanol; HEAT to dissolve in a water bath. TRANSFER
to a 125 mL separatory funnel; EXTRACT twice with 10 mL of sodium chloride
solution at 60 °C to 70 °C each time; SEPARATE (centrifuge if necessary);
COMBINE the extracts. ADD 2.0 mL of 6 mol/L hydrochloric acid solution; HEAT
in a water bath at 68 °C ~ 70 °C for 15 min; ADD methyl red indicator solution
Share