1
/
/
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.314-2020 English PDF (GB1886.314-2020)
GB 1886.314-2020 English PDF (GB1886.314-2020)
Normal fiyat
$125.00 USD
Normal fiyat
İndirimli fiyat
$125.00 USD
Birim fiyat
/
/
Kargo, ödeme sayfasında hesaplanır.
Teslim alım stok durumu yüklenemedi
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.314-2020
Historical versions: GB 1886.314-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1886.314-2020: National food safety standard - Food additive - Edetate calcium disodium
GB 1886.314-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard - Food Additive -
Edetate Calcium Disodium
食品添加剂 乙二胺四乙酸二钠钙
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People’s Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical Name, Molecular Formula, Structural Formula and Relative
Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Inspection Method ... 5
Appendix B Infrared Spectrogram of Edetate Calcium Disodium ... 11
Appendix C Standard Chromatogram of Aminotriacetic Acid and Edetate
Calcium Disodium ... 12
National Food Safety Standard - Food Additive -
Edetate Calcium Disodium
1 Scope
This Standard is applicable to food additive - edetate calcium disodium generated by
chelating disodium edetate and calcium (usually calcium carbonate or natural shells).
2 Chemical Name, Molecular Formula, Structural
Formula and Relative Molecular Mass
2.1 Chemical Name
Edetate calcium disodium
2.2 Molecular Formula
C10H12CaN2Na2O8 2H2O
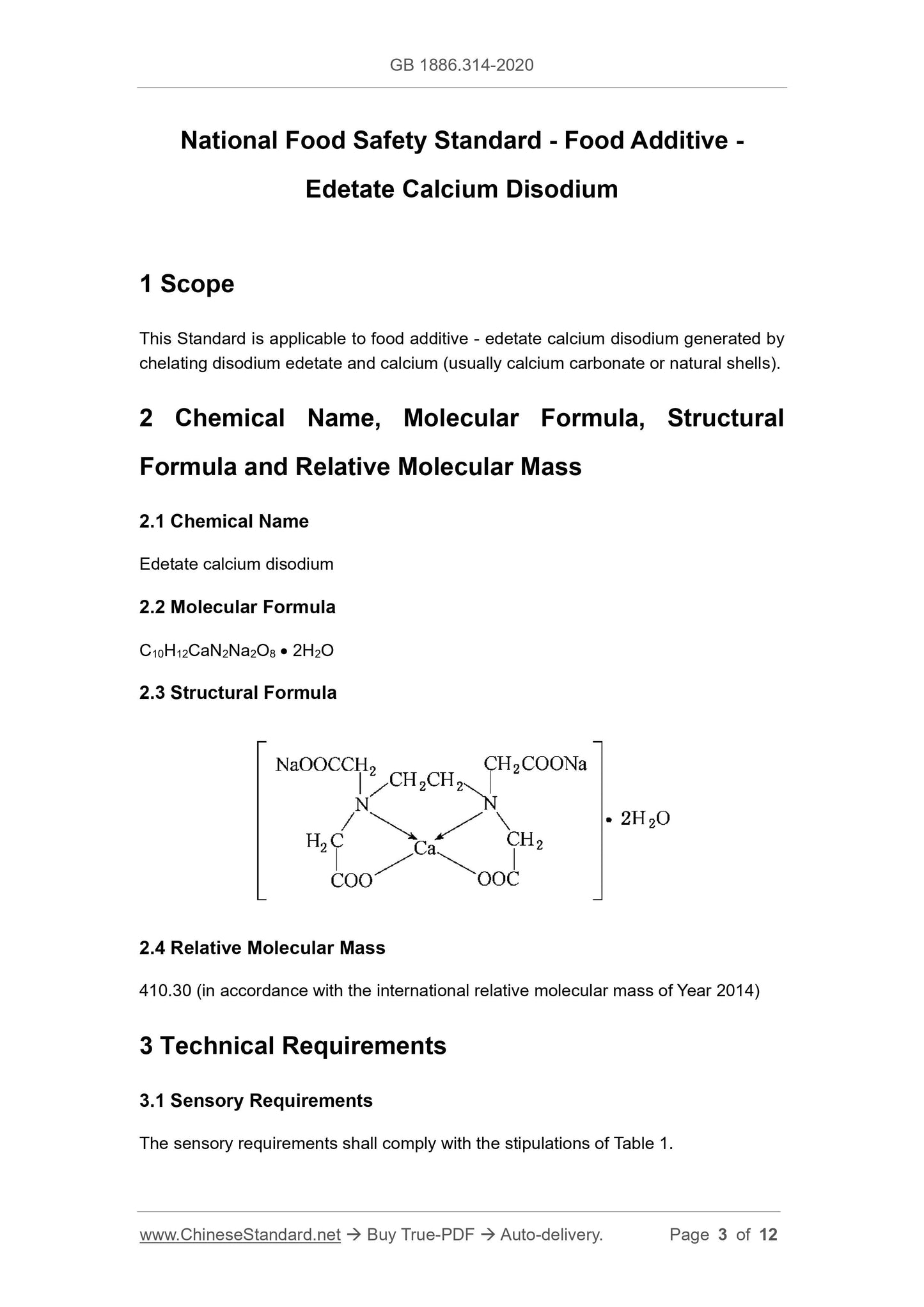
2.3 Structural Formula
2.4 Relative Molecular Mass
410.30 (in accordance with the international relative molecular mass of Year 2014)
3 Technical Requirements
3.1 Sensory Requirements
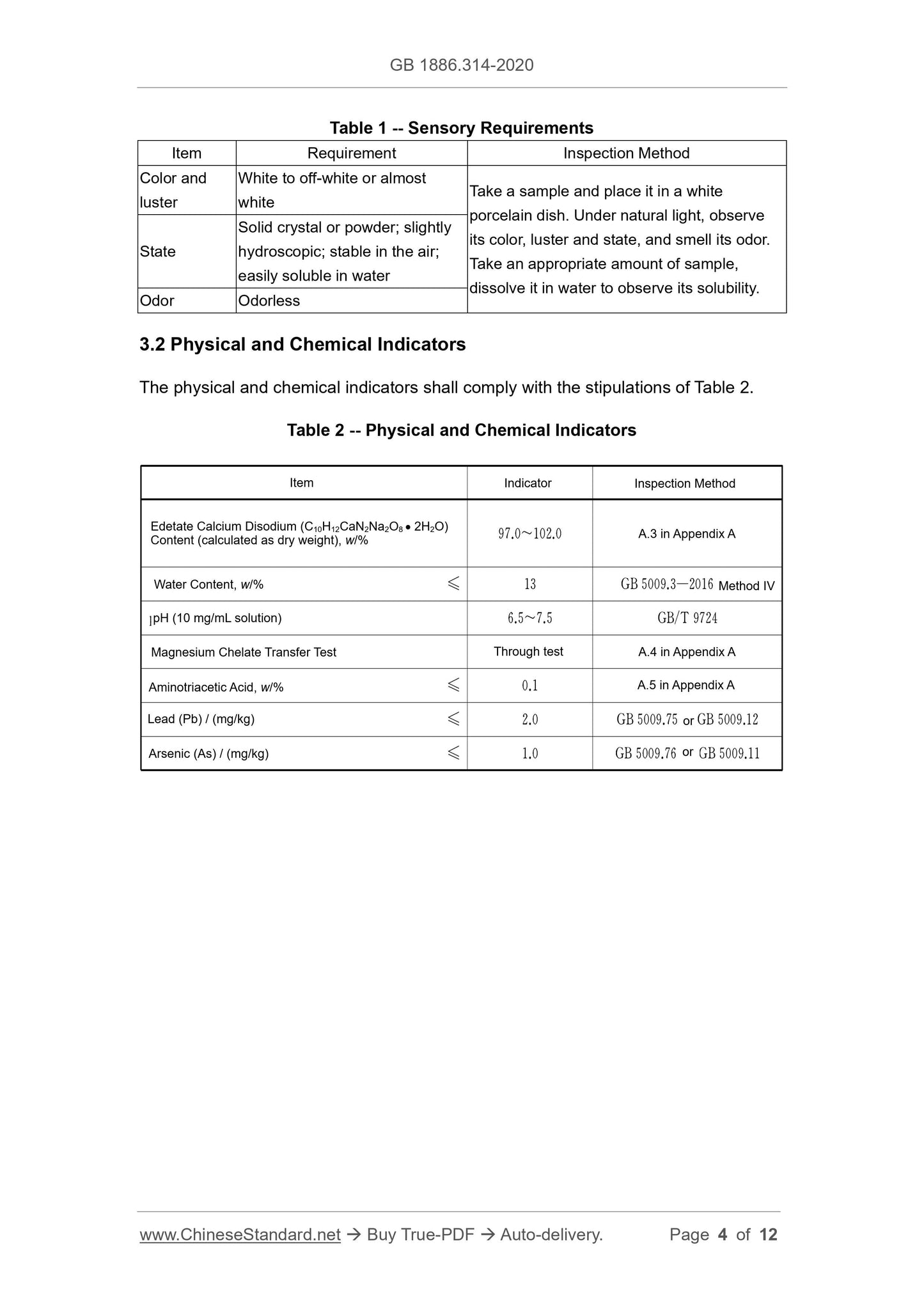
The sensory requirements shall comply with the stipulations of Table 1.
Appendix A
Inspection Method
A.1 General Provisions
When no other requirements are indicated, the reagents and water used in this
Standard refer to analytically pure reagents and Grade-3 water specified in GB/T 6682.
When no other requirements are indicated, the standard titration solutions, and
standard solutions, preparations and products for impurity determination shall be
prepared in accordance with the stipulations of GB/T 601, GB/T 602 and GB/T 603.
When the solvent used for solution preparation is not specified, it refers to aqueous
solution in the tests.
A.2 Identification Test
A.2.1 Method summary
Use the flame test to identify the sodium ions in the sample; use the oxalic acid test to
identify the calcium ions in the sample; the residual ethylene diamine tetra-acetic acid
ions in edetate calcium disodium can make the iron chloride solution fade, so as to
identify the ethylene diamine tetra-acetic acid ions. Infrared spectroscopy is a
qualitative analysis of edetate calcium disodium in accordance with the absorption
bands of the ionic groups of the constituent substances within the infrared range (mid-
infrared: 400 cm-1 ~ 4,000 cm-1).
A.2.2 Reagents and materials
A.2.2.1 Ammonium hydroxide solution (6 mol/L): measure-take 400 mL of 28%
ammonium hydroxide solution; use water to dilute it to 1,000 mL.
A.2.2.2 Ammonium oxalate solution (0.5 mol/L): take 3.5 g of ammonium oxalate
monohydrate; dissolve it in water to 100 mL.
A.2.2.3 Ammonium thiocyanate solution (0.02 g/mL): weigh-take 2 g of ammonium
thiocyanate; dissolve it in water to 100 mL.
A.2.2.4 Hydrochloric acid solution (3 mol/L): measure-take 226 mL of hydrochloric acid;
use water to dilute it to 1,000 mL.
A.2.2.5 Ferric chloride solution (1 mol/L): weigh-take 9 g of ferric chloride hexahydrate;
dissolve it in water to 100 mL.
A.2.2.6 Methyl red solution: weigh-take 100 mg of methyl red; dissolve it in 100 mL of
ethanol; if necessary, filter it.
A.2.3 Instruments and equipment
Infrared spectrophotometer.
A.2.4 Identification method
A.2.4.1 Weigh-take 0.2 g of sample; prepare a sample solution (50 mg/mL). Take
platinum wire; use hydrochloric acid solution to moisten it, then, dip it in the sample
solution. Burn it in a colorless flame; the flame is bright yellow.
A.2.4.2 Take 5 mL of the sample solution (50 mg/mL) in A.2.4.1; add 2 drops of methyl
red solution as an indicator; use ammonium hydroxide solution to adjust it to neutral.
Then, use hydrochloric acid solution to titrate the sample solution to acidic; add
ammonium oxalate solution. Thus, white precipitate - calcium oxalate is generated.
The calcium oxalate is insoluble in acetic acid, but soluble in hydrochloric acid. Use
hydrochloric acid solution to moisten it, then, dip it in the sample solution. Burn it in a
colorless flame; the flame is yellow-red.
A.2.4.3 Take 5 mL of water into a test tube. Add 2 drops of ammonium thiocyanate
solution and 2 drops of ferric chloride solution to obtain a dark red solution. Then, add
50 mg of sample. After mixing, the dark red color disappears.
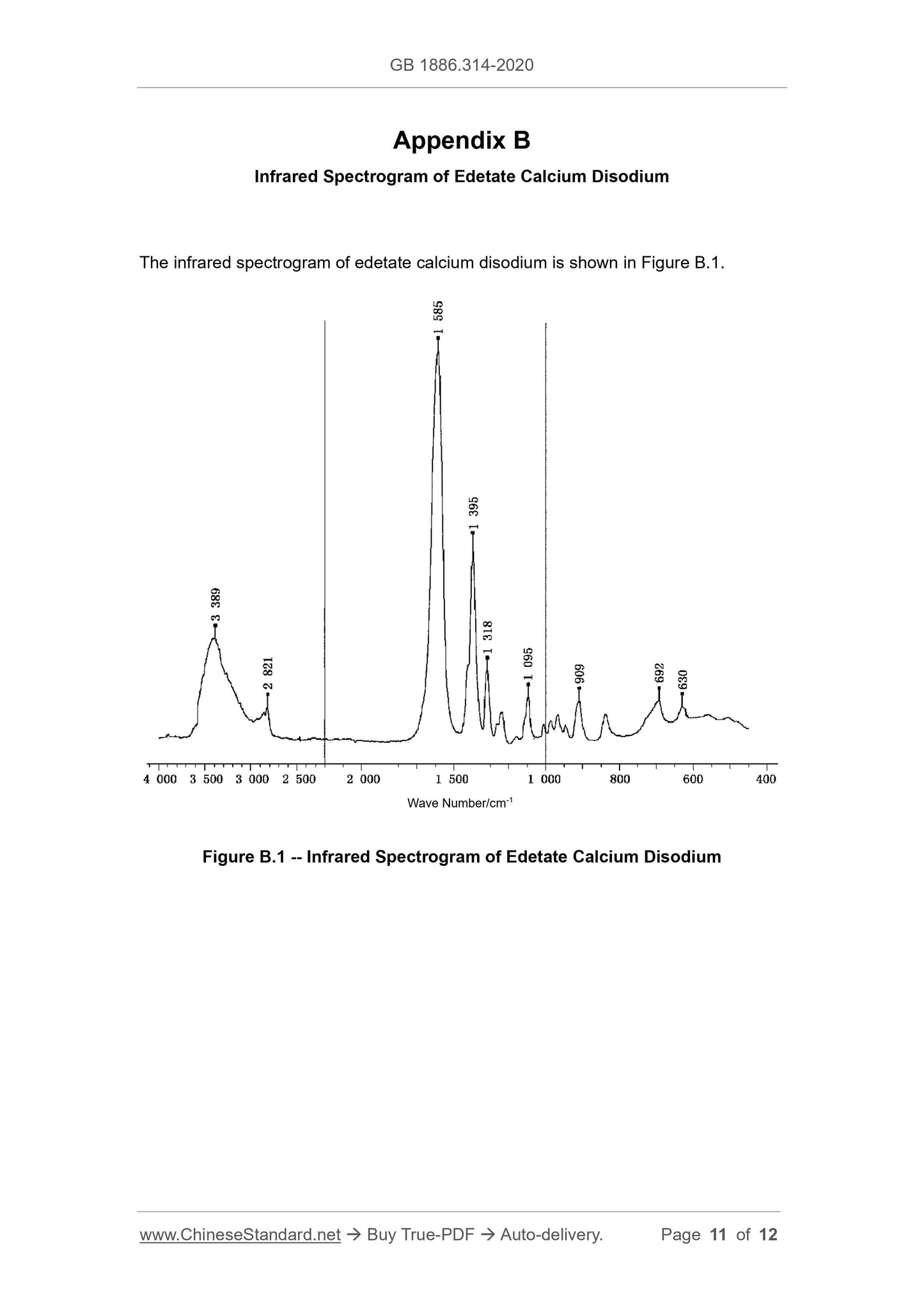
A.2.4.4 See Appendix B for the infrared spectrum of edetate calcium disodium.
A.3 Determination of Edetate Calcium Disodium (C10H12CaN2Na2O8 2H2O)
Content
A.3.1 Method summary
Edetate calcium disodium is under acidic conditions. Take diphenyl-carbazone as an
indicator; the mercury nitrate solution is titrated to purple-red as the end point. Use the
amount of consumed mercury nitrate to calculate the edetate calcium disodium content.
A.3.2 Reagents and materials
A.3.2.1 Mercury nitrate solution.
A.3.2.2 Nitric acid solution.
A.3.2.3 Ferric ammonium sulfate solution: weigh-take 8 g of ferric ammonium sulfate
dodecahydrate; dissolve it in water to 100 mL.
A.3.2.4 Ammonium thiocyanate standard titration solution (0.1 mol/L): weigh-take 8 g
of ammonium thiocyanate; dissolve it in water to 1,000 mL. In accordance with the
requirements of GB/T 601, calibrate it.
A.3.2.5 6% acetic acid solution: take 60 mL of glacial acetic acid or 166 mL of acetic
acid; use water to dilute to 1,000 mL.
V2---the volume of mercury nitrate solution consumed during the titration process,
expressed in (mL);
0.4103---each mL of mercury nitrate (1 mol/L) is equivalent to 0.4103 g of this product
(C10H12CaN2Na2O8 2H2O);
w---the mass of the sample, expressed in (g).
The test result shall be subject to the arithmetic mean value of the parallel
determination results.
A.4 Magnesium Chelate Transfer Test
A.4.1 Method summary
Under alkaline conditions, the residual ethylene diamine tetra-acetic acid free
monomers in the sample preferentially forms stable complexes with magnesium ions.
When excessive magnesium ions are complexed with acidic chrome black T, a deep
wine-red complex is formed.
A.4.2 Reagents and materials
A.4.2.1 Concentrated ammonia: 28% ammonia.
A.4.2.2 Acid mordant black test solution: weigh-take about 200 mg of acidic chrome
black T and 2 g of hydroxylamine hydrochloride; dissolve in methanol to 50 mL. Filter
it, then, store in a light-shielded container; use it within 2 weeks.
A.4.2.3 Magnesium acetate solution (0.1 mol/L): accurately weigh-take 1.1 g of
magnesium acetate tetrahydrate; dissolve it in water to 100 mL.
A.4.3 Analytical procedures
A.4.3.1 Preparation of buffer solution
Accurately weigh-take 67.5 g of ammonium chloride; dissolve it in 200 mL of water.
Then, add 570 mL of concentrated ammonia; add water to dilute to 1,000 mL.
A.4.3.2 Test
Accurately weigh-take 1 g of sample; dissolve it in 5 mL of water. Add 5 mL of buffer
solution, then, add 5 drops of the acid mordant black test solution. Use magnesium
acetate solution to titrate it, till it manifests a dark wine-red color. The required amount
of magnesium acetate solution is ≤ 2 mL.
A.5 Determination of Aminotriacetic Acid
A.5.1 Method summary
10 μg/mL.
A.5.5.4 Preparation of sample solution
Accurately weigh-take 1.0 g of the sample; transfer it to a 100 mL volumetric flask. Use
copper ni...
Get QUOTATION in 1-minute: Click GB 1886.314-2020
Historical versions: GB 1886.314-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1886.314-2020: National food safety standard - Food additive - Edetate calcium disodium
GB 1886.314-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard - Food Additive -
Edetate Calcium Disodium
食品添加剂 乙二胺四乙酸二钠钙
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People’s Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical Name, Molecular Formula, Structural Formula and Relative
Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Inspection Method ... 5
Appendix B Infrared Spectrogram of Edetate Calcium Disodium ... 11
Appendix C Standard Chromatogram of Aminotriacetic Acid and Edetate
Calcium Disodium ... 12
National Food Safety Standard - Food Additive -
Edetate Calcium Disodium
1 Scope
This Standard is applicable to food additive - edetate calcium disodium generated by
chelating disodium edetate and calcium (usually calcium carbonate or natural shells).
2 Chemical Name, Molecular Formula, Structural
Formula and Relative Molecular Mass
2.1 Chemical Name
Edetate calcium disodium
2.2 Molecular Formula
C10H12CaN2Na2O8 2H2O
2.3 Structural Formula
2.4 Relative Molecular Mass
410.30 (in accordance with the international relative molecular mass of Year 2014)
3 Technical Requirements
3.1 Sensory Requirements
The sensory requirements shall comply with the stipulations of Table 1.
Appendix A
Inspection Method
A.1 General Provisions
When no other requirements are indicated, the reagents and water used in this
Standard refer to analytically pure reagents and Grade-3 water specified in GB/T 6682.
When no other requirements are indicated, the standard titration solutions, and
standard solutions, preparations and products for impurity determination shall be
prepared in accordance with the stipulations of GB/T 601, GB/T 602 and GB/T 603.
When the solvent used for solution preparation is not specified, it refers to aqueous
solution in the tests.
A.2 Identification Test
A.2.1 Method summary
Use the flame test to identify the sodium ions in the sample; use the oxalic acid test to
identify the calcium ions in the sample; the residual ethylene diamine tetra-acetic acid
ions in edetate calcium disodium can make the iron chloride solution fade, so as to
identify the ethylene diamine tetra-acetic acid ions. Infrared spectroscopy is a
qualitative analysis of edetate calcium disodium in accordance with the absorption
bands of the ionic groups of the constituent substances within the infrared range (mid-
infrared: 400 cm-1 ~ 4,000 cm-1).
A.2.2 Reagents and materials
A.2.2.1 Ammonium hydroxide solution (6 mol/L): measure-take 400 mL of 28%
ammonium hydroxide solution; use water to dilute it to 1,000 mL.
A.2.2.2 Ammonium oxalate solution (0.5 mol/L): take 3.5 g of ammonium oxalate
monohydrate; dissolve it in water to 100 mL.
A.2.2.3 Ammonium thiocyanate solution (0.02 g/mL): weigh-take 2 g of ammonium
thiocyanate; dissolve it in water to 100 mL.
A.2.2.4 Hydrochloric acid solution (3 mol/L): measure-take 226 mL of hydrochloric acid;
use water to dilute it to 1,000 mL.
A.2.2.5 Ferric chloride solution (1 mol/L): weigh-take 9 g of ferric chloride hexahydrate;
dissolve it in water to 100 mL.
A.2.2.6 Methyl red solution: weigh-take 100 mg of methyl red; dissolve it in 100 mL of
ethanol; if necessary, filter it.
A.2.3 Instruments and equipment
Infrared spectrophotometer.
A.2.4 Identification method
A.2.4.1 Weigh-take 0.2 g of sample; prepare a sample solution (50 mg/mL). Take
platinum wire; use hydrochloric acid solution to moisten it, then, dip it in the sample
solution. Burn it in a colorless flame; the flame is bright yellow.
A.2.4.2 Take 5 mL of the sample solution (50 mg/mL) in A.2.4.1; add 2 drops of methyl
red solution as an indicator; use ammonium hydroxide solution to adjust it to neutral.
Then, use hydrochloric acid solution to titrate the sample solution to acidic; add
ammonium oxalate solution. Thus, white precipitate - calcium oxalate is generated.
The calcium oxalate is insoluble in acetic acid, but soluble in hydrochloric acid. Use
hydrochloric acid solution to moisten it, then, dip it in the sample solution. Burn it in a
colorless flame; the flame is yellow-red.
A.2.4.3 Take 5 mL of water into a test tube. Add 2 drops of ammonium thiocyanate
solution and 2 drops of ferric chloride solution to obtain a dark red solution. Then, add
50 mg of sample. After mixing, the dark red color disappears.
A.2.4.4 See Appendix B for the infrared spectrum of edetate calcium disodium.
A.3 Determination of Edetate Calcium Disodium (C10H12CaN2Na2O8 2H2O)
Content
A.3.1 Method summary
Edetate calcium disodium is under acidic conditions. Take diphenyl-carbazone as an
indicator; the mercury nitrate solution is titrated to purple-red as the end point. Use the
amount of consumed mercury nitrate to calculate the edetate calcium disodium content.
A.3.2 Reagents and materials
A.3.2.1 Mercury nitrate solution.
A.3.2.2 Nitric acid solution.
A.3.2.3 Ferric ammonium sulfate solution: weigh-take 8 g of ferric ammonium sulfate
dodecahydrate; dissolve it in water to 100 mL.
A.3.2.4 Ammonium thiocyanate standard titration solution (0.1 mol/L): weigh-take 8 g
of ammonium thiocyanate; dissolve it in water to 1,000 mL. In accordance with the
requirements of GB/T 601, calibrate it.
A.3.2.5 6% acetic acid solution: take 60 mL of glacial acetic acid or 166 mL of acetic
acid; use water to dilute to 1,000 mL.
V2---the volume of mercury nitrate solution consumed during the titration process,
expressed in (mL);
0.4103---each mL of mercury nitrate (1 mol/L) is equivalent to 0.4103 g of this product
(C10H12CaN2Na2O8 2H2O);
w---the mass of the sample, expressed in (g).
The test result shall be subject to the arithmetic mean value of the parallel
determination results.
A.4 Magnesium Chelate Transfer Test
A.4.1 Method summary
Under alkaline conditions, the residual ethylene diamine tetra-acetic acid free
monomers in the sample preferentially forms stable complexes with magnesium ions.
When excessive magnesium ions are complexed with acidic chrome black T, a deep
wine-red complex is formed.
A.4.2 Reagents and materials
A.4.2.1 Concentrated ammonia: 28% ammonia.
A.4.2.2 Acid mordant black test solution: weigh-take about 200 mg of acidic chrome
black T and 2 g of hydroxylamine hydrochloride; dissolve in methanol to 50 mL. Filter
it, then, store in a light-shielded container; use it within 2 weeks.
A.4.2.3 Magnesium acetate solution (0.1 mol/L): accurately weigh-take 1.1 g of
magnesium acetate tetrahydrate; dissolve it in water to 100 mL.
A.4.3 Analytical procedures
A.4.3.1 Preparation of buffer solution
Accurately weigh-take 67.5 g of ammonium chloride; dissolve it in 200 mL of water.
Then, add 570 mL of concentrated ammonia; add water to dilute to 1,000 mL.
A.4.3.2 Test
Accurately weigh-take 1 g of sample; dissolve it in 5 mL of water. Add 5 mL of buffer
solution, then, add 5 drops of the acid mordant black test solution. Use magnesium
acetate solution to titrate it, till it manifests a dark wine-red color. The required amount
of magnesium acetate solution is ≤ 2 mL.
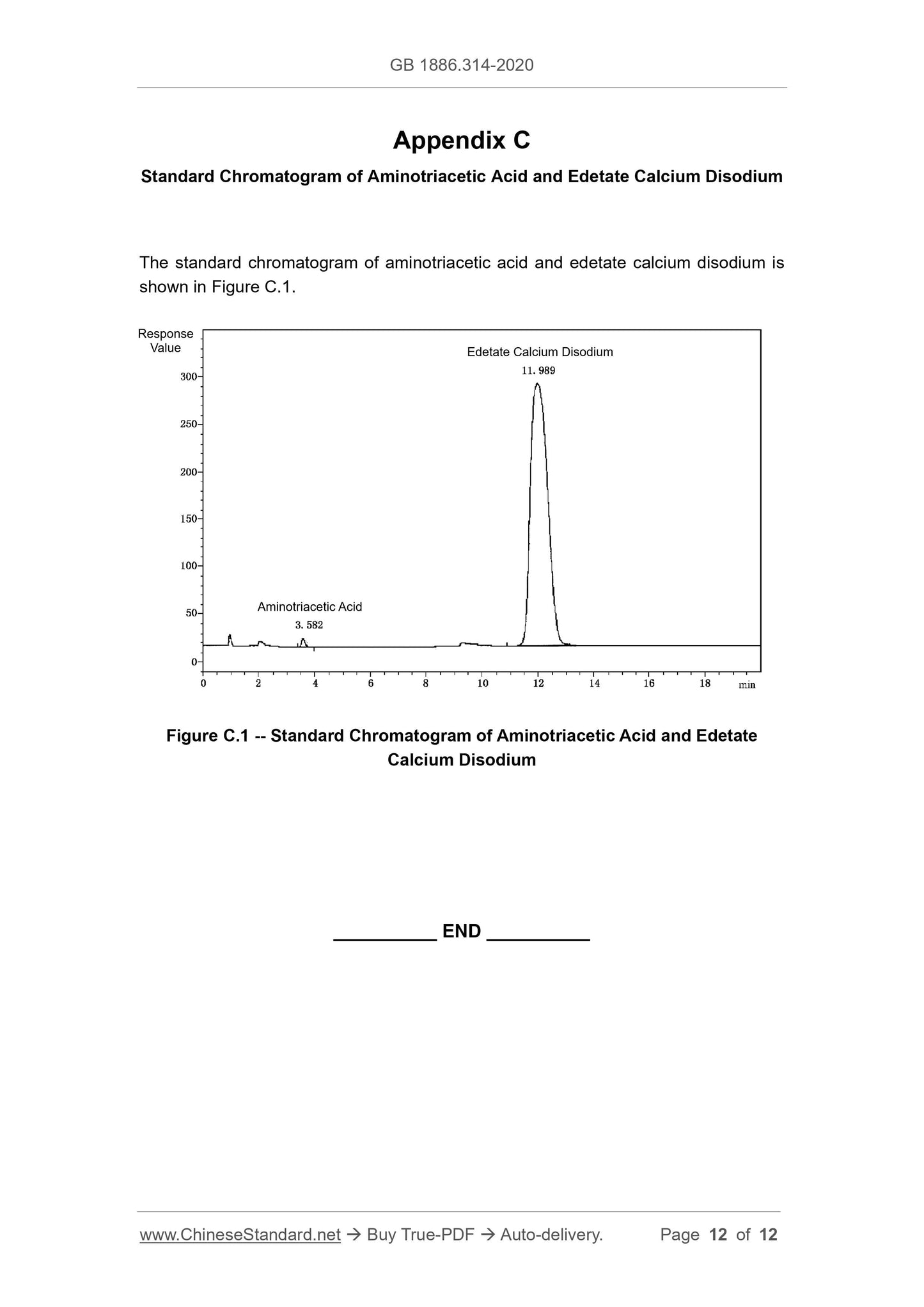
A.5 Determination of Aminotriacetic Acid
A.5.1 Method summary
10 μg/mL.
A.5.5.4 Preparation of sample solution
Accurately weigh-take 1.0 g of the sample; transfer it to a 100 mL volumetric flask. Use
copper ni...
Share