1
/
/
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.320-2021 English PDF (GB1886.320-2021)
GB 1886.320-2021 English PDF (GB1886.320-2021)
Normal fiyat
$110.00 USD
Normal fiyat
İndirimli fiyat
$110.00 USD
Birim fiyat
/
/
Kargo, ödeme sayfasında hesaplanır.
Teslim alım stok durumu yüklenemedi
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.320-2021
Historical versions: GB 1886.320-2021
Preview True-PDF (Reload/Scroll if blank)
GB 1886.320-2021: National food safety standard - Food additives - Sodium gluconate
GB 1886.320-2021
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food additives -
Sodium gluconate
ISSUED ON: FEBRUARY 22, 2021
IMPLEMENTED ON: AUGUST 22, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, molecular formula, structural formula and relative molecular
mass ... 3
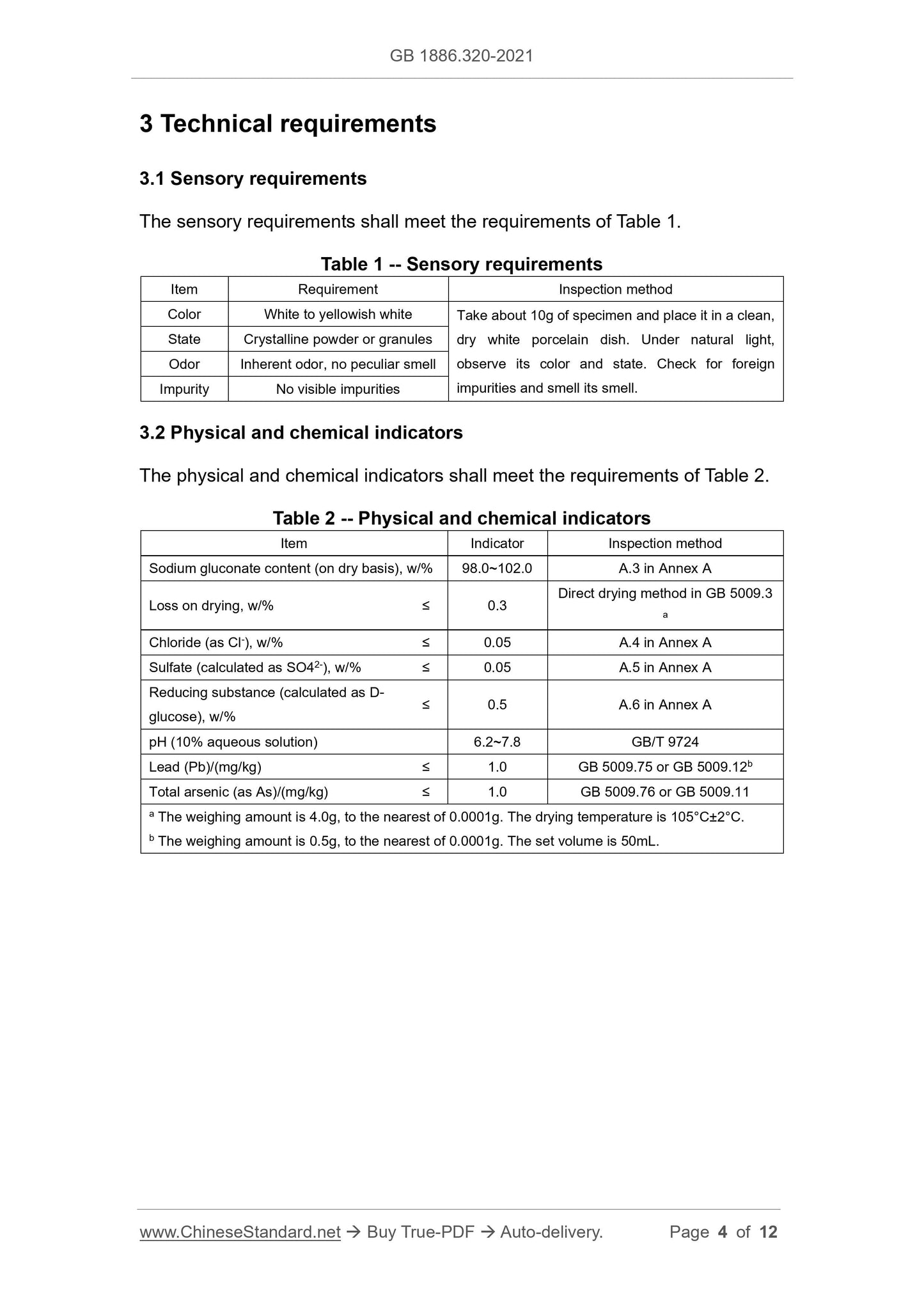
3 Technical requirements ... 4
Annex A Inspection methods ... 5
National food safety standard - Food additives -
Sodium gluconate
1 Scope
This Standard is applicable to the food additive sodium gluconate. It takes
gluconic acid and sodium hydroxide produced by starch fermentation as main
raw materials, and is concentrated, crystallized and dried through the chemical
reaction.
2 Chemical name, molecular formula, structural
formula and relative molecular mass
2.1 Chemical name
Sodium gluconate.
2.2 Molecular formula
C6H11NaO7
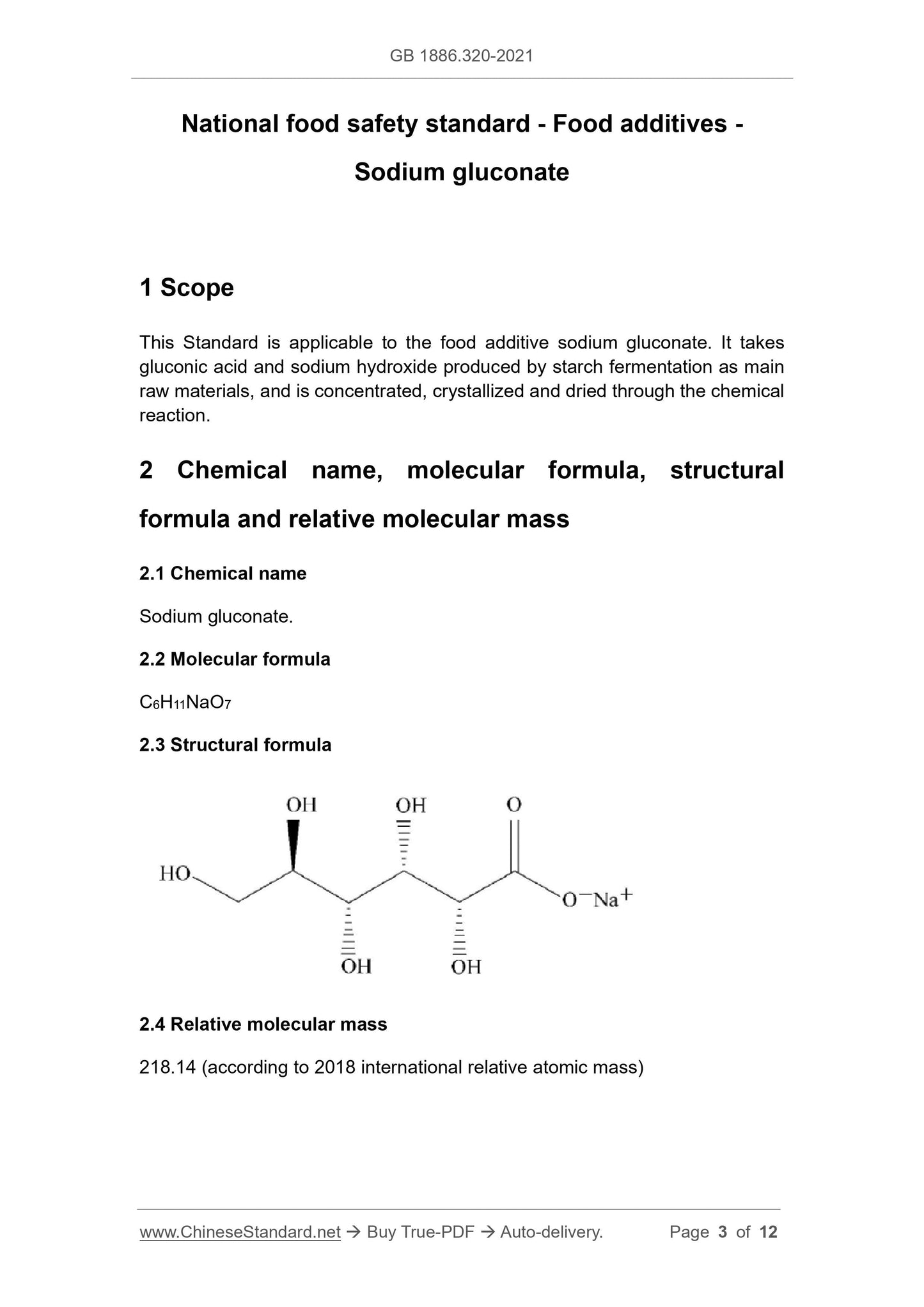
2.3 Structural formula
2.4 Relative molecular mass
218.14 (according to 2018 international relative atomic mass)
Annex A
Inspection methods
WARNING - Some test procedures specified by the test method may lead
to dangerous situations. The operator shall take appropriate safety and
protective measures.
A.1 General
All reagents and water used in this Standard when other requirements are not
specified, refer to analytically-pure reagents and grade three water specified in
GB/T 6682. In the test, all the standard solutions used, the standard solutions
for impurity determination, preparations and products when other requirements
are not specified, are prepared according to GB/T 601, GB/T 602, GB/T 603.
The solutions used in the test refers to aqueous solutions when it is not
specified which solvents are used to prepare.
A.2 Identification test
A.2.1 Reagents and materials
A.2.1.1 Concentrated hydrochloric acid.
A.2.1.2 Glacial acetic acid.
A.2.1.3 Ethanol.
A.2.1.4 Phenylhydrazine.
A.2.1.5 Platinum wire.
A.2.2 Instruments and equipment
A.2.2.1 Water bath.
A.2.2.2 Electronic balance: Resolution is 0.01g.
A.2.3 Identification method
A.2.3.1 Identification of sodium ion
A.2.3.1.1 Method principle
According to the phenomenon that sodium ions burn on a colorless flame and
the flame is bright yellow, identify the presence of sodium ions.
acetic acid (if necessary, use an electric hot plate to slightly heat). Add 2~3
drops of crystal violet indicator solution. Use perchloric acid standard titration
solution to titrate till the solution changes from purple to blue and finally to green,
which shall be the end point. Except for no specimen, use the same amount of
reagent solution for blank test. When in use, the temperature of the perchloric
acid standard titrant shall be the same as the temperature at the time of
calibration. If the temperature difference is less than 4°C, the concentration of
the perchloric acid standard titration solution shall be corrected to the
concentration at the operating temperature. If the temperature difference is
greater than 4°C, it shall be re-calibrated.
A.3.1.5 Result calculation
The mass fraction w1 of sodium gluconate (calculated as C6H11NaO7) is
calculated according to formula (A.1).
Where,
V1 - The volume of perchloric acid standard titration solution consumed by the
specimen solution, in milliliters (mL);
V0 - The volume of perchloric acid standard titration solution consumed by the
blank solution, in milliliters (mL);
c1 - The actual concentration of perchloric acid standard titration solution after
temperature correction, in moles per liter (mol/L);
M1 - The molar mass of sodium gluconate, in grams per mole (g/mol)
(M1=218.14);
m1 - The mass of specimen after the loss on drying is determined, in grams (g);
1000 - The conversion factor.
The calculation result retains three significant figures.
Take the arithmetic mean of the parallel measurement results as the
measurement result. The absolute difference between the two parallel
determination results is not more than 0.3%.
A.3.2 Potentiometric titration method
A.3.2.1 Method principle
c1 - The actual concentration of perchloric acid standard titration solution after
temperature correction, in moles per liter (mol/L);
M1 - The molar mass of sodium gluconate, in grams per mole (g/mol)
(M1=218.14);
m1 - The mass of specimen weighed after determining the loss on drying, in
grams (g);
1000 - The conversion factor.
The calculation result retains three significant figures. Take the arithmetic mean
of the parallel measurement results as the measurement result. The absolute
difference between the two parallel determination results is not more than 0.3%.
A.4 Determination of chloride (as Cl-)
A.4.1 Method principle
Under acidic conditions, the chloride ion in the sodium gluconate solution and
the silver nitrate solution form a white silver chloride precipitate. Compare the
turbidity with the standard solution by visual inspection.
A.4.2 Reagents and materials
A.4.2.1 Nitric acid solution: Measure 105mL of nitric acid. Use water to set
volume to 1000mL.
A.4.2.2 Silver nitrate solution: 17g/L. Accurately weigh 17.0g of silver nitrate.
Use water to dissolve and set volume to 1000mL.
A.4.2.3 Chloride standard solution: 0.1mg/mL. Prepare according to GB/T 602.
A.4.3 Instruments and equipment
Electronic balance: Resolution is 0.01g and 0.0001g.
A.4.4 Analysis steps
A.4.4.1 Preparation of specimen solution
Weigh 0.4g of specimen, to the nearest of 0.01g. Place in a 50mL Nessler
colorimetric tube. Add 20mL of water to dissolve. Use it as the specimen
solution.
A.4.4.2 Preparation of standard solution
Pipette 2.00mL of sodium chloride standard solution. Place in a 50mL Nessler
colorimetric tube. Use 20mLof water to dilute. Use it as the standard solution.
hydrochloric acid solution and 5mL of barium chloride solution. Separately use
water to set volume to 50mL. Shake well slowly. Place for 10min.
Place the two on a black background. Look down from the top of the colorimetric
tube. Compare the turbidity produced. The turbidity of the sample solution is
not deeper than the turbidity of the standard solution, that is, the sulfate in the
specimen is not more than 0.05%.
A.6 Determination of reducing substances (as D-glucose)
A.6.1 Method principle
The reducing sugar reduces the divalent copper ion to cuprous oxide. The
remaining divalent copper ions react with iodide ions under acidic conditions to
generate quantitative iodine. Titrate the generated iodine with sodium
thiosulfate standard solution, so as to calculate the content of reducing sugar
in the specimen.
A.6.2 Reagents and materials
A.6.2.1 Preparation of alkaline copper citrate solution
Solution A: Weigh 173g of sodium citrate (sodium citrate) and 100g of
anhydrous sodium carbonate. Add warm water around 40°C to dissolve into
700mL (if the solution is turbid, filter to make it clear).
Solution B: Weigh 17.3g of copper sulfate pentahydrate. Add water to make it
dissolved to 100mL.
Take 100mL of solution B before use. Keep shaking. Slowly add 700mL of
solution A. After cooling, add water to set volume to 1000mL.
A.6.2.2 Iodine standard titration solution: c(1/2I2)=0.1mol/L.
A.6.2.3 Sodium thiosulfate standard titration solution: c=0.1mol/L.
A.6.2.4 Starch indicator solution: 10g...
Get QUOTATION in 1-minute: Click GB 1886.320-2021
Historical versions: GB 1886.320-2021
Preview True-PDF (Reload/Scroll if blank)
GB 1886.320-2021: National food safety standard - Food additives - Sodium gluconate
GB 1886.320-2021
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food additives -
Sodium gluconate
ISSUED ON: FEBRUARY 22, 2021
IMPLEMENTED ON: AUGUST 22, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, molecular formula, structural formula and relative molecular
mass ... 3
3 Technical requirements ... 4
Annex A Inspection methods ... 5
National food safety standard - Food additives -
Sodium gluconate
1 Scope
This Standard is applicable to the food additive sodium gluconate. It takes
gluconic acid and sodium hydroxide produced by starch fermentation as main
raw materials, and is concentrated, crystallized and dried through the chemical
reaction.
2 Chemical name, molecular formula, structural
formula and relative molecular mass
2.1 Chemical name
Sodium gluconate.
2.2 Molecular formula
C6H11NaO7
2.3 Structural formula
2.4 Relative molecular mass
218.14 (according to 2018 international relative atomic mass)
Annex A
Inspection methods
WARNING - Some test procedures specified by the test method may lead
to dangerous situations. The operator shall take appropriate safety and
protective measures.
A.1 General
All reagents and water used in this Standard when other requirements are not
specified, refer to analytically-pure reagents and grade three water specified in
GB/T 6682. In the test, all the standard solutions used, the standard solutions
for impurity determination, preparations and products when other requirements
are not specified, are prepared according to GB/T 601, GB/T 602, GB/T 603.
The solutions used in the test refers to aqueous solutions when it is not
specified which solvents are used to prepare.
A.2 Identification test
A.2.1 Reagents and materials
A.2.1.1 Concentrated hydrochloric acid.
A.2.1.2 Glacial acetic acid.
A.2.1.3 Ethanol.
A.2.1.4 Phenylhydrazine.
A.2.1.5 Platinum wire.
A.2.2 Instruments and equipment
A.2.2.1 Water bath.
A.2.2.2 Electronic balance: Resolution is 0.01g.
A.2.3 Identification method
A.2.3.1 Identification of sodium ion
A.2.3.1.1 Method principle
According to the phenomenon that sodium ions burn on a colorless flame and
the flame is bright yellow, identify the presence of sodium ions.
acetic acid (if necessary, use an electric hot plate to slightly heat). Add 2~3
drops of crystal violet indicator solution. Use perchloric acid standard titration
solution to titrate till the solution changes from purple to blue and finally to green,
which shall be the end point. Except for no specimen, use the same amount of
reagent solution for blank test. When in use, the temperature of the perchloric
acid standard titrant shall be the same as the temperature at the time of
calibration. If the temperature difference is less than 4°C, the concentration of
the perchloric acid standard titration solution shall be corrected to the
concentration at the operating temperature. If the temperature difference is
greater than 4°C, it shall be re-calibrated.
A.3.1.5 Result calculation
The mass fraction w1 of sodium gluconate (calculated as C6H11NaO7) is
calculated according to formula (A.1).
Where,
V1 - The volume of perchloric acid standard titration solution consumed by the
specimen solution, in milliliters (mL);
V0 - The volume of perchloric acid standard titration solution consumed by the
blank solution, in milliliters (mL);
c1 - The actual concentration of perchloric acid standard titration solution after
temperature correction, in moles per liter (mol/L);
M1 - The molar mass of sodium gluconate, in grams per mole (g/mol)
(M1=218.14);
m1 - The mass of specimen after the loss on drying is determined, in grams (g);
1000 - The conversion factor.
The calculation result retains three significant figures.
Take the arithmetic mean of the parallel measurement results as the
measurement result. The absolute difference between the two parallel
determination results is not more than 0.3%.
A.3.2 Potentiometric titration method
A.3.2.1 Method principle
c1 - The actual concentration of perchloric acid standard titration solution after
temperature correction, in moles per liter (mol/L);
M1 - The molar mass of sodium gluconate, in grams per mole (g/mol)
(M1=218.14);
m1 - The mass of specimen weighed after determining the loss on drying, in
grams (g);
1000 - The conversion factor.
The calculation result retains three significant figures. Take the arithmetic mean
of the parallel measurement results as the measurement result. The absolute
difference between the two parallel determination results is not more than 0.3%.
A.4 Determination of chloride (as Cl-)
A.4.1 Method principle
Under acidic conditions, the chloride ion in the sodium gluconate solution and
the silver nitrate solution form a white silver chloride precipitate. Compare the
turbidity with the standard solution by visual inspection.
A.4.2 Reagents and materials
A.4.2.1 Nitric acid solution: Measure 105mL of nitric acid. Use water to set
volume to 1000mL.
A.4.2.2 Silver nitrate solution: 17g/L. Accurately weigh 17.0g of silver nitrate.
Use water to dissolve and set volume to 1000mL.
A.4.2.3 Chloride standard solution: 0.1mg/mL. Prepare according to GB/T 602.
A.4.3 Instruments and equipment
Electronic balance: Resolution is 0.01g and 0.0001g.
A.4.4 Analysis steps
A.4.4.1 Preparation of specimen solution
Weigh 0.4g of specimen, to the nearest of 0.01g. Place in a 50mL Nessler
colorimetric tube. Add 20mL of water to dissolve. Use it as the specimen
solution.
A.4.4.2 Preparation of standard solution
Pipette 2.00mL of sodium chloride standard solution. Place in a 50mL Nessler
colorimetric tube. Use 20mLof water to dilute. Use it as the standard solution.
hydrochloric acid solution and 5mL of barium chloride solution. Separately use
water to set volume to 50mL. Shake well slowly. Place for 10min.
Place the two on a black background. Look down from the top of the colorimetric
tube. Compare the turbidity produced. The turbidity of the sample solution is
not deeper than the turbidity of the standard solution, that is, the sulfate in the
specimen is not more than 0.05%.
A.6 Determination of reducing substances (as D-glucose)
A.6.1 Method principle
The reducing sugar reduces the divalent copper ion to cuprous oxide. The
remaining divalent copper ions react with iodide ions under acidic conditions to
generate quantitative iodine. Titrate the generated iodine with sodium
thiosulfate standard solution, so as to calculate the content of reducing sugar
in the specimen.
A.6.2 Reagents and materials
A.6.2.1 Preparation of alkaline copper citrate solution
Solution A: Weigh 173g of sodium citrate (sodium citrate) and 100g of
anhydrous sodium carbonate. Add warm water around 40°C to dissolve into
700mL (if the solution is turbid, filter to make it clear).
Solution B: Weigh 17.3g of copper sulfate pentahydrate. Add water to make it
dissolved to 100mL.
Take 100mL of solution B before use. Keep shaking. Slowly add 700mL of
solution A. After cooling, add water to set volume to 1000mL.
A.6.2.2 Iodine standard titration solution: c(1/2I2)=0.1mol/L.
A.6.2.3 Sodium thiosulfate standard titration solution: c=0.1mol/L.
A.6.2.4 Starch indicator solution: 10g...
Share